Main Content
LOEWE Diffusible Signals

How do bacteria communicate with human inflammatory cells? This topic is at the center of the LOEWE research cluster "Diffusible Signals" (Impact of diffusible signals at human cell-microbe interfaces). The Hessian state government is funding "Diffusible Signals" in the 13th season of the Hessian state offensive for the development of scientific and economic excellence ("Landesoffensive zur Entwicklung wissenschaftlicher und ökonomischer Exzellenz" - LOEWE) with a total of about 4.8 million euros.
Prof. Bernd Schmeck is involved in the subprojects A2 and B1.
Subproject A2 - Diffusible Signals as Colonization Factors
Cholera is a devastating intestinal disease caused by the bacterium Vibrio cholerae. Contrary to a long-held belief, V. cholerae has recently been shown to trigger an inflammatory response during infection. However, it is unclear how V. cholerae cells interact with the innate immune system. In preliminary work, we have investigated the interaction with macrophages and found that the diffusible protein TcpF secreted by V. cholerae is essential for the binding of V. cholerae to macrophages and may have an influence on the secretion of diffusible cytokines. Here, we want to elucidate the mechanism by which TcpF can cause or facilitate the attachment of V. cholerae to macrophages and how macrophages react to TcpF. Understanding the detailed mechanism of this important step in the interaction between V. cholerae and macrophages could lead to new therapeutic approaches against cholera.
Subproject B1 - Extracellular Vesicles in Host-Microbe-Interactions
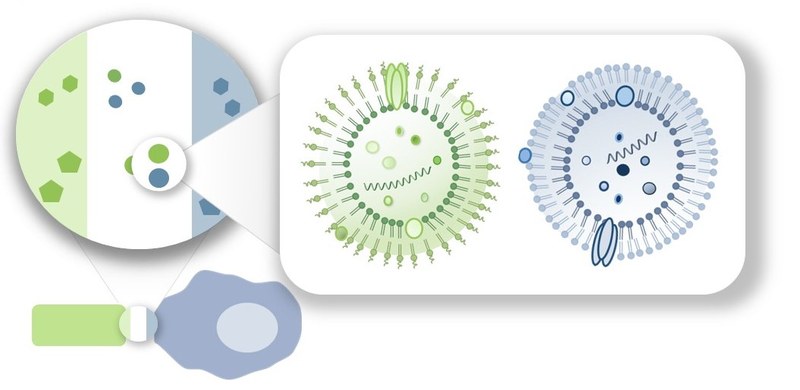
Recent results indicate that both the extracellular vesicles (EVs) of host cells and the outer membrane vesicles (OMVs) of microbes may have an influence on the course of inflammation and infection. Our own preliminary work has shown cell-specific inflammatory effects of EVs. In addition, both replication-promoting and replication-inhibiting properties of Legionella OMVs have been observed.
The aim of project B1 is to comprehensively characterize the functional components and effects of both diffusible vesicle types using the model of Klebsiella (Kp)-macrophage (MΦ) interaction. By analyzing the origin and composition of extracellular vesicles in the infection context as well as the response of host cells and bacteria thereto (recognition, uptake, utilization, signal transduction) we will significantly broaden our understanding of cell and infection biology. At the same time, the project will also develop the basis for a clinical-translational use of vesicles in diagnostics, prevention and therapy of bacterial infectious diseases.