Main Content
Abstracts of the talks presented at the Ladies' Day of FOR 2824 on May 20th, 2021
Prof. Dr. Anke KruegerInstitut für Organische Chemie |

|
Nanoscale Diamond, a Material for Many ApplicationsNanodiamond is a carbon allotrope with unique properties. It combines the unusual characteristics of diamond such as mechanical and chemical stability, biocompatibility and negative electron affinity with the properties of a nanoscale materials such as specific chemical reactivity. This lecture will introduce the synthesis, properties, functionalization and characterization of functional nanodiamond materials and will give examples for applications in biomedicine, imaging and catalysis. |
|
Dr. Anita Zeidler
Physics Department |

|
Order within DisorderAmorphous materials are characterised by their inherent disorder. Atoms in the materials (e.g. liquids or glasses) are seemingly randomly distributed throughout. |
|
Prof. Dr. Barbara KirchnerMulliken Center for Theoretical Chemistry |

|
Chirality and Proton activity from molecular to ionic liquids |
|
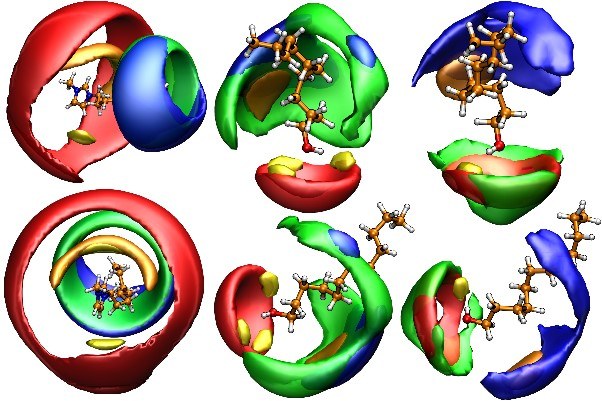
|
Ionic liquids (ILs) [1] are playing a role in many exciting applications as and for materials.[2] They are used in so different areas as in electrochemistry, catalysis, in coal processing, in pharmaceutical applications.[2] Understanding which one of all the possibilities is the right ionic liquid to use is the key to the successful outcome of the particular applications. Therefore, an understanding of the molecular level behavior,[3] of the given liquid itself is desirable if the full potential of the solvent should be reached. For example, the structure-directing or template effect [4,5,6] has been invoked several times for ILs to explain the different outcome in material synthesis when varying the IL. We will first discuss chirality and its detection,[9] which plays an important role for the understanding of pharmaceuticals in ILs or pharmaceutical ILs. [10] Second, we focus on electrolyte applications of ILs. In such systems, the association of the ions to form ion pairs or other, low charge aggregates is a long discussed issue,[7] since it can affect the manner and the extent of conduction through changing the number of mobile charged species in the solution. Structural diffusion in ILs [8] can be connected to electrochemical applications. In particular, the proton transfer ability of solvents is a highly important feature for electrolytes and for solvents in synthesis as well. |
References [1] P. Wasserscheid, P. and Welton T., eds. Ionic liquids in synthesis. John Wiley & Sons, 2008. |
|
Prof. Dr. Katja HeinzeDepartment of Chemistry |

|
Spin-Flip Emission with Earth-Abundant Metal IonsThe development of metal complexes with Earth-abundant first row transition metal ions as emitters for (PH)OLEDs, as dyes for DSSCs and sensor devices or as photocatalysts is very challenging, due to the typically fast non-radiative deactivation of the electronically excited states.[1] Beyond the classically exploited luminescent charge transfer states, metal-centered states with small excited state distortion can become emissive. This is realized in so-called spin-flip emitters.[1,2] |
|
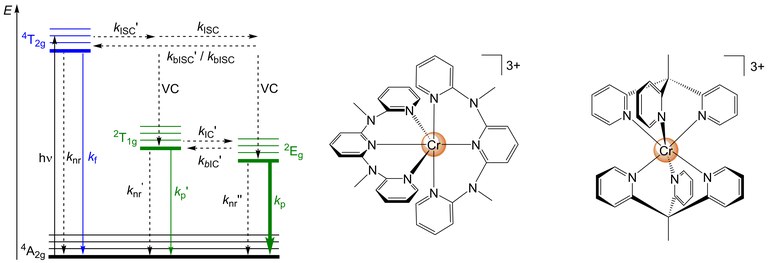
|
Figure 1. Jablonski diagram with relevant photophysical processes of octahedral d3-metal complexes and “molecular rubies” [Cr(ddpd)2]3+ [3] and [Cr(tpe)2]3+ [8]. |
|
[1] C. Förster, K. Heinze, Chem. Soc. Rev. 2020, 49, 1057. |
|
Prof. Dr. Stefanie GräfeInstitute of Physical Chemistry and Institute of Applied Physics |

|
Plasmonic hybrid systems in external light fields: can we achieve sub-nanometer lateral resolution using near-field techniques?What is the ultimate spatial resolution that can be achieved with near-field methods? In current experiments, for example based on tip-amplified Raman scattering (TERS), there is increasing evidence for an extremely high spatial resolution on the nanometer or even sub-nanometer scale. In this talk, I will present some of these experiments of our collaboration partner, Prof. Volker Deckert from Jena, as well as the first results of our calculations. |
|
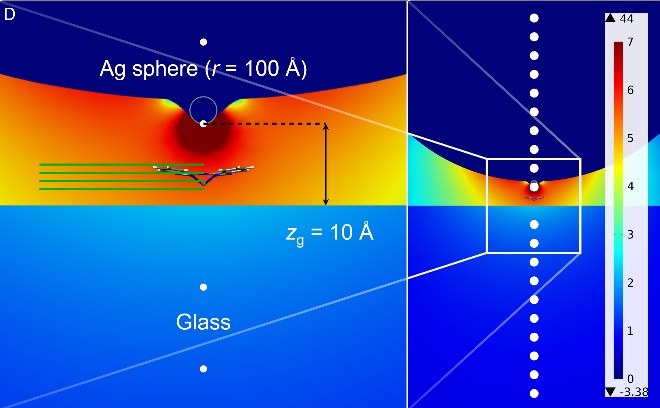
|
|
|
References: |
|
|
|
|
|
|
|

