Hauptinhalt
Revealing the Reaction Pathway of Anodic Hydrogen Evolution at Magnesium Surfaces in Aqueous Electrolytes
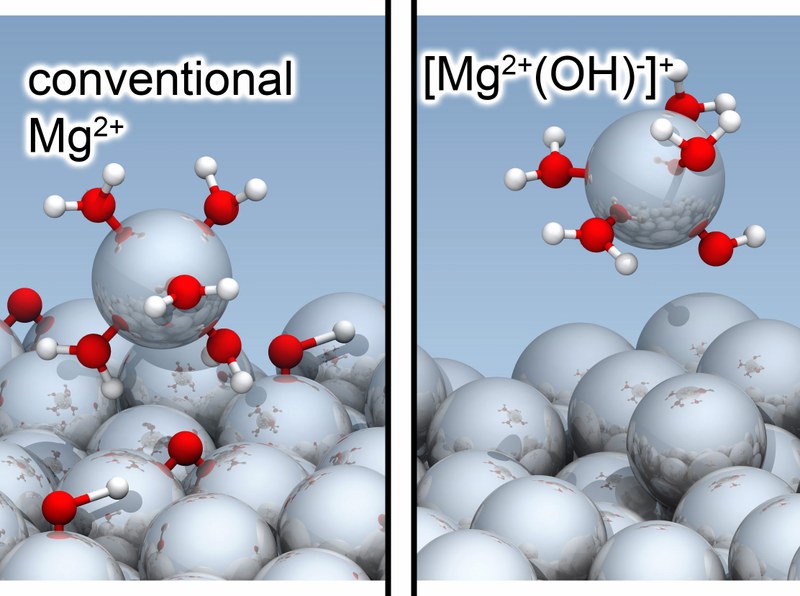
Aqueous metal corrosion is a major economic concern in modern society. A phenomenon that has puzzled generations of scientists in this field is the so-called anomalous hydrogen evolution: magnesium, prized for its light weight, dissolves violently under electron-deficient (anodic) conditions, accompanied by strong hydrogen evolution. This effect is a key mechanism hampering Mg technology. Experimental studies have indicated the presence of univalent Mg+ in solution, but these findings have been largely ignored because they defy our common chemical understanding and evaded direct experimental observation. Using recent advances in the ab initio description of solid-liquid electrochemical interfaces under controlled potential conditions, the groups of Prof. Wippermann and Prof. Neugebauer (MPI Düsseldorf) traced the full reaction path of Mg atom dissolution under anodic conditions and uncover a surprising twist: the research reveals that magnesium instead forms a unique [Mg²⁺(OH)⁻]⁺ complex, explaining the elusive Mg⁺ ions observed experimentally and the absence of protective oxide layers on corroding magnesium. This discovery paves the way for new anti-corrosion strategies for magnesium and other metals. The work is published in the Journal of the American Chemical Society (JACS).