Hauptinhalt
Research

The activation and transformation of strong bonds by complexes with earth abundant first-row transition metals is one of the primary pathways towards a more sustainable chemistry. To achieve this goal we are combining unusual oxidation states with low-coordinate complex geometries to obtain highly reactive metal centres with unusual electronic and chemical properties.
Linear metal(I/II) complexes
Quasilinear, monovalent 3d-transition metal complexes are a fascinating class of molecules, that combine such an uncommon coordination geometry with an for a 3d-transition metal unusual oxidation state. We develop a broad synthetic entry using mainly silylamide but also NHC ligands. By that we could already we obtain a number of linear metal(I/II) compounds of Cr - Ni, that are modulated by sterics or introduction of functional groups. These compounds can show exceptional single-molecule magnetic behaviour which origins in the oxidation state/geometry combination. We further shape the elcetronic profile by introduction of other donor functionalities (e.g. phosphinidenes with AK v. Hänisch, Marburg).
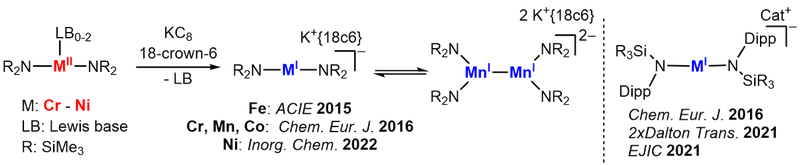
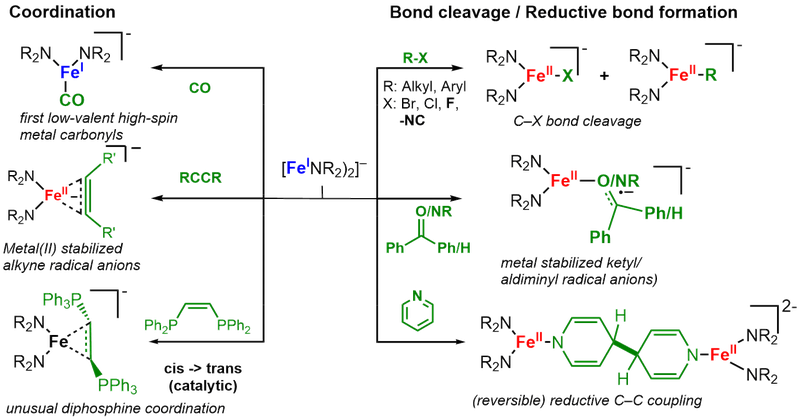
Subsequently their reactivity towards different bond types and small molecules is examined to map out broadly for the first time the reactivity patters of these linear metal(I) complexes. As such we could isolate the first low-valent high-spin metal carbonyl, and observe cleavage of C-F bonds or reversible reductive coupling of pyridine. Furthermore it leads to the observation of unique metal stabilized radical anion - such as alkinyl, alkenyl or ketiminyl radical anions.
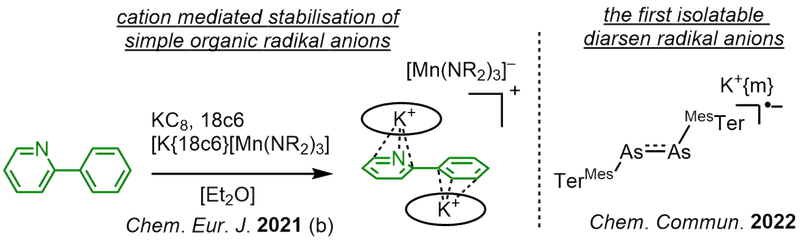
Radical Anions
Radical anions play an important role in organic transformations and main group chemistry, yet are notoriously reactive and hard to isolate. Building on our expertise on metal stabilized radical anions we pursue a novel synthetic approaches to isolate such "free" radical anions void of transition metal complexation. This is achieved by encapsualtion between to cations that allowed for the isolation of prototypical stilbene or pyridine radical anions. This allows for precise insights into their chemical behaviour on a synethically accessible level. The handling of highly sensitive organic radical anions extends also to heavier mainggroup systems, and allow for the first example of a diarsene radical anion (coop. with AK Hering-Junghans, LIKAT Rostock).
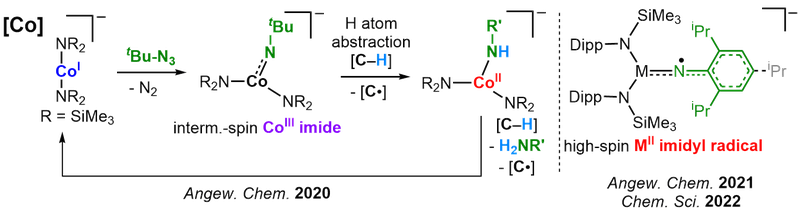
Low-coordinate late-3d-metal imido complexes in higher spin states
The metal-catalyzed amination of unfunctionalized C–H bonds is an atom economic approach to secondary amines. It is usually described as the formal insertion of of a metal bound nitrene into the C–H bond. Due to the generally high-reactivity of late 3d-metal imido complexes the electronic and geometric factors that drive their reactivity and the involved reaction mechanisms are ill understood. As a general rule high-spin complexes tend to be more reactive, whereas most isolated complexes are found in a low-spin state. Using low-coordiante metal platforms we want to shed light on the complete reaction cascade of amination reactions, starting from interaction of the metal with the nitrene transfer agent, the imido metal unit itself and its reactivity, as well as the involvement of the potentially formed metal amido species. By variation of oxidation and spin states, metals (Cr - Cu) as well as imido substituents we want to map out the reactivity patterns within a low-coordinate environment, with the ultimate goal of predicting and steering C–H amination catalysis. In this quest we could obtain the first cobalt imide that facilitates H atom abstraction from external substrates, the first imidyl cobalt complex, as well as an imido iron complex with mixed imidyl and nitrene character.
Low-coordinate 3d-metal/maingroup clusters
Last but not least we are concerned with low-coordinate 3d-metal/main group clusters. This ranges from bio-inspired iron/sulfur clusters to stabilisation and solubilsation of Zintl-ions (coop. with AK Dehnen, INT Karlsruhe).
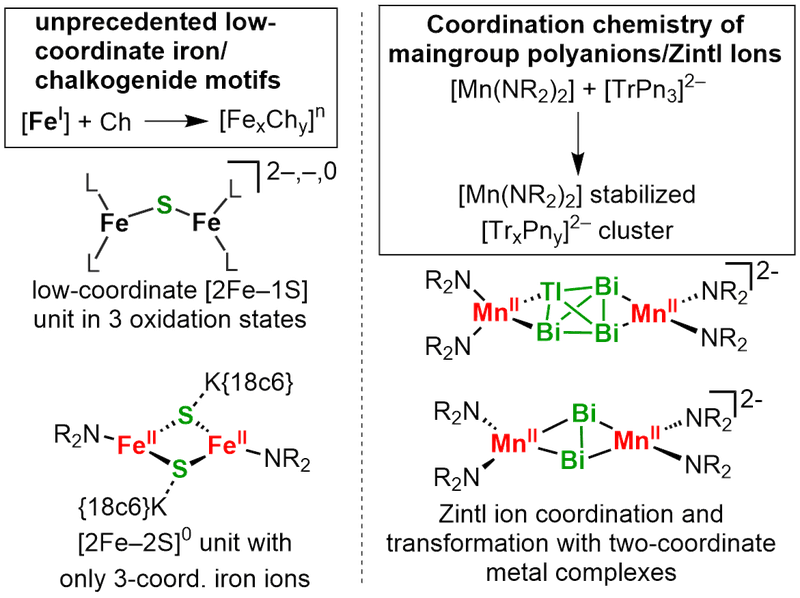
For these studies we use a variety of analytic techniques that are available either "in-house" or with the help of cooperation partners (low-temeprature paramagnetic NMR-, EPR-, XAS, 57Fe-Mössbauer- and UV/Vis spectroscopy; mass spectrometry; magnetic measurements using dc- and ac-SQUID; quantum chemical calculations).