Main Content
Area A - Chemosensoring
A1 - Diffusible Signals and bacterial Motility
PI: Victor Sourjik
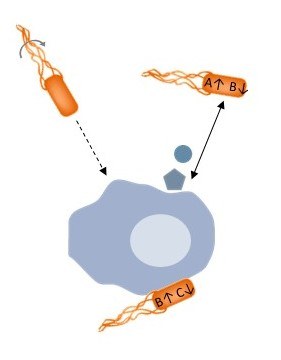
Subproject A1 investigates the hypothesis that diffusible signals have an important influence on the interactions between bacteria (both commensal and pathogenic E. coli and S. enterica ssp. I ser. Typhimurium) and host cells (monocytes and macrophages) by chemotaxis and control of bacterial motility. The interactions between floating bacteria and monocytes or macrophages are monitored microscopically in real time and the chemotactic response of the bacteria to the diffusible messengers excreted by monocytes or macrophages is studied. In case of recognized chemo effectors, the bacterial receptors are identified and the signaling mechanisms are characterized. Furthermore, the regulation of bacterial gene expression is determined by the substances secreted by the monocytes or macrophages and by direct contact with their surfaces.
Although many pathogenic bacteria are mobile and have a chemotaxis system, the exact meaning of motility and chemotaxis in the interaction between pathogenic bacteria and their host remains largely unclear. This interaction will be investigated on several levels, from the perception of chemical signals to gene regulation, on a molecular and mechanistic level. A1 will contribute to the general understanding of the infection mechanisms of pathogenic bacteria and lead to the development of preventive therapies based on chemotaxis inhibitors.
A2 - Diffusible Signals as Colonization Factors
PI: Knut Drescher
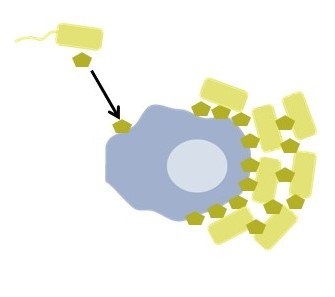
Cholera is a devastating intestinal disease caused by the bacterium Vibrio cholerae. Contrary to a long-held belief, V. cholerae has recently been shown to trigger an inflammatory response during infection. However, it is unclear how V. cholerae cells interact with the innate immune system. In preliminary work, we have investigated the interaction with macrophages and found that the diffusible protein TcpF secreted by V. cholerae is essential for the binding of V. cholerae to macrophages and may have an influence on the secretion of diffusible cytokines. Here, we want to elucidate the mechanism by which TcpF can cause or facilitate the attachment of V. cholerae to macrophages and how macrophages react to TcpF. Understanding the detailed mechanism of this important step in the interaction between V. cholerae and macrophages could lead to new therapeutic approaches against cholera.
A3 - Machine Learning for Antimicrobial Peptides
PI: Dominik Heider
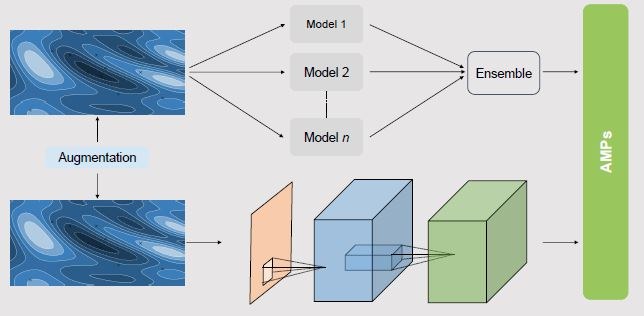
Antimicrobial peptides are important diffusible signals in the interaction of host cells with a bacterium. In subproject A3 we aim to develop models for the prediction of antimicrobial peptides (AMPs) and to identify AMPs from the immune response of cells during infection. AMPs exist in almost all organisms as part of the immune system and have achieved very good results in laboratory experiments, especially with multi-resistant pathogens. AMPs act selectively on certain pathogens and have two known mechanisms of action: first, they destroy the bacterial membrane, second, they penetrate the bacterial cells and inhibit the DNA/RNA and protein biosynthesis of the pathogens. In the in vitro and murine infection models, peptides will first be identified by mass spectrometry, bioinformatically optimized, their usability for antibiotic treatment computer-assisted and finally the effectiveness of selected constructs will be tested experimentally. For this purpose, methods of multi-objective optimization and machine learning, especially from the field of classifier ensembles, multi-label learning and deep learning, will be used and further developed accordingly. The methods for computer-aided analysis and optimization of AMPs developed here can be used for the development of new peptide-based antibiotics with our industrial partners.




