Main Content
Subcellular trafficking of galectin-3
Glycan chains can be identified by sugar binding proteins termed lectins, which are classified according to their sequence and structural homology. The family of galectins recognizes β galactosides by means of a highly conserved carbohydrate recognition domain (CRD) and consists of 15 family members, which are categorized by their domain composition. This galectin-division into prototype, tandem repeat and chimeric type was introduced in 1993 by Hirabayashi and Kasai. Prototype galectins comprising galectins 1, 2, 5, 7, 11, 13, 14 and 15, contain a single CRD and act as monomers or dimers. Galectins of the tandem repeat type (galectins 4, 6, 8, 9 and 12) possess two covalently linked homologous but not identical CRDs. Galectin-3 is the only chimeric type variant in vertebrates and consists of a CRD and a proline- and glycine-rich N terminal domain. This lectin is synthesized in the cytosol is secreted independently of the endoplasmatic recticulum (ER) into the extracellular milieu.
In epithelial cells, apical protein transport is heterogeneous and involves at least two distinct transport pathways based on the dependence or independence of lipid rafts as transport platform. Galectin-3 was identified as sorting receptor to recruit lipid raft-independent apical cargo molecules into apical transport vesicles. Galectin-3 accumulation in the lumen of intracellular vesicles was not expected initially since the lectin is not secreted by the secretory pathway but uses non-conventional secretion. Recently, we identified a late domain motif in the N-terminal domain of galectin-3 that recruits the lectin into multivesicular bodies for non-classical exosomal secretion into the apical milieu. Once at the apical membrane, galectin-3 reenters the cell by endocytosis and traverses endosomes from where it is recycled back to the cell surface. This recycling is modulated by the pH-gradient between the extracellular milieu and endosomal organelles.
We are currently working on further elucidation of galectin-3 recycling.
Galectin-3 recycling in MDCK cells.
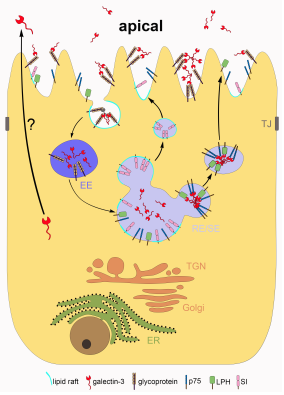
Galectin-3 is synthesized on ribosomes in the cytosol and released by an unkown unconventional secretion mechanism into the extracellular milieu. On the apical cell surface galectin-3 assembles with glycoproteins into lattices that are at least partially co-stained by caveolin or flotillin. Following endocytosis, the lattice can disassemble in acidic early endosomal compartments (dark purple). Released galectin-3 converges with newly synthesized cargo from the TGN in sorting endosomes to form high molecular weight clusters at elevated pH for recruitment into raft-independent vesicles destined for the apical membrane. ER, endoplasmic reticulum; TGN, trans Golgi network; EE, early endosome; RE/ SE, recycling/ sorting endosome; TJ, tight junction.