Main Content
Thermal electro-poling of solid-state materials
Thermal electro-poling describes a technique used for manipulating specific properties of solid-state materials. The technique is simple. Place a sample of interest between to metal electrodes and apply a DC potential drop across the sample. If the temperature is high enough some native ions will become mobile and a concentration profile evolves. The process of poling is in general associated with an exponentially decreasing poling current. Once the current as dropped to zero, poling is finished and the changes brought about can be frozen by cooling to room temperature.
Electro-poling receives attention due to possible applications in non-linear optics and in artificial bone replacements.
While the translocation of ions has been known for a long time, the role of electron mobility has been controversial for a long time. Some years ago we presented a full model explaining and rationalizing the concentration depth profiles arising during electro-poling. The crucial point in the model is, that translocation of cations away from the anode gives rise to an increase in the local electric field trying to stop the cation transport. This is the standard view of a situation leading to a blocking of ion transport. What has been often neglected, is, that the electric field may become so high, that it reaches the dielectric breakdown field strength of the material. In that situation electrons become mobile and become ejected into the anode. As a consequence a depletion zone is formed, which is not only empty of mobile cations but also empty of the corresponding number of electrons. The external potential drops across this depletion zone. The blocking of charge carrier transport in this cases arises from the fact that no potential is left to drop across the bulk of the sample [1].
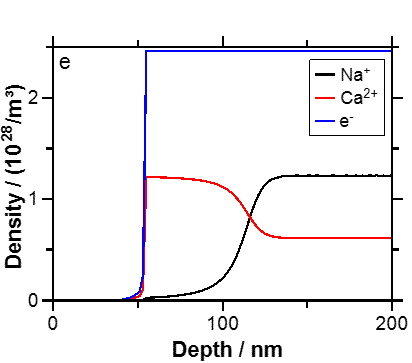
Fig. 1 Concentration depth profile of Na+, Ca++ and electrons at the end of the poling of the bio-active glass 46S4.
Thermal electro-poling can be applied to many ion conducting materials. We have e.g. reported the poling of D263T glass [2].
Literature:
[1] M. Schäfer, K.-M. Weitzel, Solid State Ionics, 282, 70–75, (2015)
Numerical model for electro-poling
[2] K. Rein, M. Schäfer, and K.-M. Weitzel,
The role of dieelectric breakdown in electro-thermal poling of D263T glass
IEEE Transactions on dielectrics and electrical insulation, p. 1422, (2020)
DOI