Main Content
Publications
PUBLICATIONS IN PEER-REVIEWED JOURNALS
21. Homoleptic Bismuth Alkynes: Isolable Reagents for Selective Alkynyl Radical Transfer.
Kundu, G.; Debbeler, F.; Reith, S.; Wurm, F.; Casitas, A.; Lichtenberg, C.*
Angew. Chem. Int. Ed. 2026, 65, e19525. DOI: 10.1002/anie.202519525.
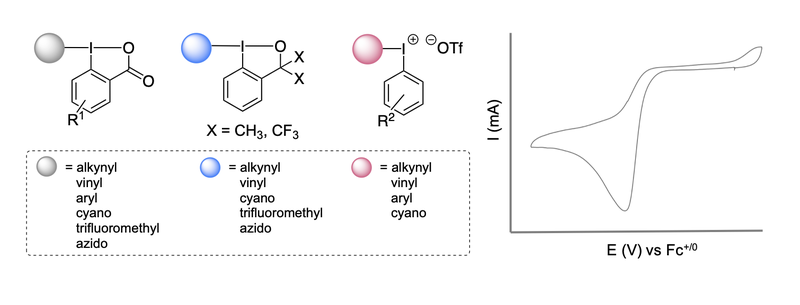
20. Experimental electrochemical potentials of iodine(III) reagents.
Debbeler, F.;# Jenisch, D.;# Souilah, C.; Moths, P.; Ivlev, S.; Casitas, A.* (# equal contribution)
Synthesis 2025, 57, 2946-2962. DOI: 10.1055/a-2650-7789
19. Synthesis of Fe(IV) Alkynylide Complexes and Their Reactivity to Form 1,3-diynes.
Souilah, C.;# Jannuzzi, S. A. V.;# Becker, F. J.; Demirbas, D.; Jenisch, D.; Ivlev S., Xie, X; Peredkov, S; Lichtenberg, C.; DeBeer, S.*, Casitas, A.* (# equal contribution)
Angew. Chem. Int. Ed. 2025, 64, e202421222. DOI: 10.1002/anie.20242122299

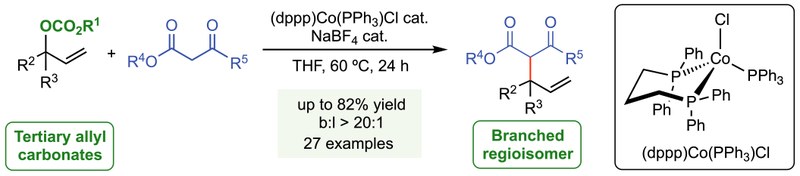
18. Experimental and Computational Studies on Cobalt(I)-Catalyzed Regioselective Allylic Alkylation Reactions.
Andreetta, P.; Martin, R. T.; Souilah, C.; Rentería-Gómez, A.; Zhihui Song, Z.; Khorramshahi Bayat, Y.; Ivlev, S.; Gutierrez, O.*; Casitas, A.*
Angew. Chem. Int. Ed. 2023, 62, e202310129. DOI: 10.1002/anie.202310129
- Highlighted in Organic Process Research & Development (OPR&D) from ACS Editorial. Org. Process Res. Dev. 2023, 27, 1848−1857. DOI: 10.1021/acs.oprd.3c00389
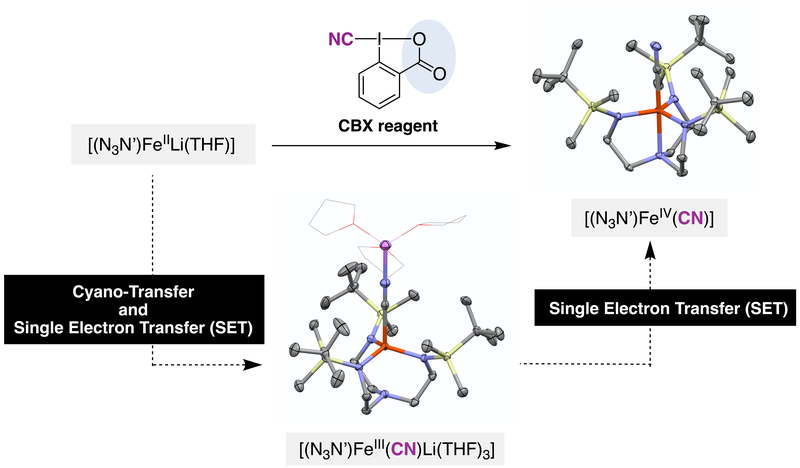
17. Synthesis of Fe(III) and Fe(IV) cyanide complexes using hypervalent iodine(III) reagents as cyano-transfer one-electron oxidants.
Souilah, C.; Jannuzzi, S. A. V.; Demirbas, D.; Ivlev, S.; Swart, M.; DeBeer, S.; Casitas A.*
Angew. Chem. Int. Ed. 2022, 61, e202201699. DOI: 10.1002/anie.202201699
- Not peer-reviewed: Introducing... Alicia Casitas Angew. Chem. Int. Ed. 2022. DOI: 10.1002/anie.202204754
BOOK CHAPTERS
5. Iron-catalyzed group-transfer reactions with iodine(III) reagents. Casitas, A.*; Andreetta, P. Book chapter Advances in Catalysis, vol. 74, p.33-98: Earth-Abundant Transition Metal Catalyzed Reactions. Ed: M.Diéguez and T. Ollevier. 2024. Elsevier, ISBN: 978-0-443-14003-7.
NON-PEER REVIEW CONTRIBUTIONS
4. A reflection on recent advances in organometallic copper(III) chemistry
Casitas, A.;* Ribas, X.* Chem. Sci. 2026, 17, 694-698. DOI: 10.1039/D5SC90259B.
3. Light-driven strategies for stereocenter editing
Casitas, A.; Medhi, K. Nachrichten aus der Chemie 2025, 73, issue 12, 58-63. DOI: 10.1002/nadc.20254153841.
2. Enzyme Zähmen Radikale
Casitas, A.; Debbeler F. Nachrichten aus der Chemie 2025, 73, issue 7-8, 58-63. (Sprache auf Deutsch)
DOI: 10.1002/nadc.20254152322.
1. Safer and cheaper ways to carbenes
Casitas, A.; Becker F. J. Nachrichten aus der Chemie 2025, 73, issue 5, 80-84.
DOI: 10.1002/nadc.20254147306.
Publications prior to Philipps-Universität Marburg
PUBLICATIONS IN PEER-REVIEWED JOURNALS
16. Light-Driven Reduction of Aromatic Olefins in Aqueous Media Catalysed by Aminopyridine Cobalt Complexes.
Casadevall, C.; Pascual, D.; Aragón, J.; Call, A.; Casitas, A.; Casademont-Reig, I., Lloret-Fillol, J.*
Chem. Sci. 2022, 13, 4270-4282. DOI: 10.1039/D1SC06608K
15. Visible light Reductive Cyclization of Nonactivated Alkyl Chlorides.
Claros, M.; Casitas, A.*; Lloret-Fillol,J.*
Synlett 2019, 30, 1496–1507. (*corresponding author) DOI: 10.1055/s-0037-1611878
14. Reductive Cyclization of Unactivated Alkyl Chlorides with Tethered Alkenes under Visible-Light Photoredox Catalysis.
Claros, M.; Ungeheuer, F.; Franco, F.; Martin-Diaconescu, M.; Casitas, A.*; Lloret-Fillol,J.*
Angew. Chem. Int. Ed. 2019, 58, 4869-4874. (*corresponding author) DOI: 10.1002/anie.201812702.
13. Ligand exchange on an Allylic C-H Activation by Iron(0) Fragments: π-complexes, Allyliron Species and Metallacycles.
Casitas, A., Krause, H., Lutz, S., Bill, E., Goddard, R.; Fürstner, A.*
Organometallics 2018, 37, 729-739. DOI: 10.1021/acs.organomet.7b00571.
12. Two Exceptional Homoleptic Iron(IV) Tetraalkyl Complexes.
Casitas, A., Rees, J., Goddard, R., Bill, E., DeBeer, S., Fürstner A.*
Angew. Chem. Int. Ed. 2017, 56, 10108-10113. DOI: 10.1002/anie.201612299.
- Highlighted in Nature Reviews Chemistry 2017, DOI: 10.1038/s41570-017-0036
11. Dual cobalt-copper light-driven catalytic reduction of aldehydes and aromatic ketones in aqueous media.
Call, A., Casadevall, C., Acuña-Parés, F., Casitas, A., Lloret-Fillol, J.*
Chem. Sci. 2017, 8, 4739-4749. (Cover of the Journal) DOI: 10.1039/C7SC01276D.
10. Elementary Steps of Iron Catalysis: Exploring the Links between Iron Alkyl and Iron Olefin Complexes for their Relevance in C-H activation and C-C Bond Formation.
Casitas, A., Krause, H., Goddard, R., Fürstner, A.*
Angew. Chem. Int. Ed. 2015, 54, 1521-1526. DOI: 10.1002/anie.201410069.
9. Cu-Catalyzed Cascades to Carbocycles: Union of Diaryliodonium Salts with Alkenes or Alkynes Exploiting Remote Carbocations.
Zhang, F., Das, S., Walkinshaw, A. J., Casitas, A., Taylor, M., G. Suero, M. G., Gaunt, M. J.*
J. Am. Chem. Soc. 2014, 136, 8851-8854. DOI: 10.1021/ja504361y.
8. The role of organometallic copper(III) complexes in homogeneous catalysis.
Casitas, A., Ribas, X.*
Chem. Sci. 2013, 4, 2301-2318. DOI: 10.1039/C3SC21818J.
7. Aryl-O reductive elimination from reaction of well-defined aryl-CuIIIspecies with phenolates: the importance of ligand reactivity.
Casitas, A., Ioannidis, N., Mitrikas, G.,* Costas, M., Ribas, X.*
Dalton Trans. 2011, 40, 8796-8799. DOI: 10.1039/C1DT10428D.
6. Observation and mechanistic study of facile C–O bond formation between a well-defined aryl-copper(III) complex and oxygen nucleophiles.
Huffman, L. M., Casitas, A.*, Font, M., Canta, M., Costas, M., Ribas, X.,* Stahl, S. S.*
Chem. Eur. J. 2011, 17, 10643-10650. (*equal contribution) DOI: 10.1002/chem.201100608
5. Nucleophilic aryl fluorination and aryl halide exchange mediated by a CuI/CuIII catalytic cycle.
Casitas, A., Canta, M., Solà, M., Costas, M., Ribas, X.*
J. Am. Chem. Soc. 2011, 133, 19386-19392. DOI: 10.1021/ja2058567.
4. Copper-Catalyzed Aerobic Oxidative Functionalization of an Arene C-H Bond: Evidence for an Aryl-Copper(III) Intermediate.
King, A. E., Huffman, L. M., Casitas, A., Costas, M., Ribas, X.,* Stahl, S. S.*
J. Am. Chem. Soc. 2010, 132, 12068-12073. DOI: 10.1021/ja1045378.
3. Direct observation of CuI/CuIIIredox steps relevant to Ullmann-type coupling reactions.
Casitas, A., King A. E., Parella, T., Costas, M., Stahl,* S. S., Ribas, X.*
Chem. Sci. 2010, 1, 326-330. DOI: 10.1039/C0SC00245C.
2. Molecular mechanism of acid-triggered aryl-halide cross-coupling reaction via reductive elimination in well-defined aryl-CuIII-halide species.
Casitas, A., Poater, A., Solà, M., Stahl, S. S., Costas, M., Ribas, X.*
Dalton Trans. 2010, 39, 10458-10463. DOI: 10.1039/C0DT00284D.
1. Facile C-H Bond Cleavage via a Proton-Coupled Electron Transfer Involving a C-H···CuII interaction.
Ribas, X.,* Calle, C., Poater, A., Casitas, A., Gómez, L., Xifra, R., Parella, T., Benet-Buchholz, J., Schweiger, A., Mitrikas,* G., Solà,* M., Llobet, A.,* Stack, T. D. P.*
J. Am. Chem. Soc. 2010, 132, 12299-12306. DOI: 10.1021/ja101599e.
BOOK CHAPTERS
4. Halide Exchange Reactions Mediated by Transition Metals. Casitas, A.* 11, pp. 275-293 in “Discovering the Future of Molecular Sciences”,.Ed.: B. Pignataro, Wiley-VCH, 2014. ISBN 978-3-5273-3544-2.
3. Aromatic/Vinylic Finkelstein Reaction. Casitas, A., Ribas, X.* 6, pp. 239-252 in “Copper-Mediated Cross-Coupling Reactions”, Ed.: G. Evano, N. Blanchard. Wiley, 2013. ISBN 978-1-118-06045-2.
2. Insights into the Mechanism of Modern Ullmann–Goldberg Coupling Reactions. Casitas, A., Ribas, X.* 7, pp. 253-280 in “Copper-Mediated Cross-Coupling Reactions”, Ed.: G. Evano, N. Blanchard. Wiley, 2013. ISBN 978-1-118-06045-2.
1. The Bioinorganic and Organometallic Chemistry of Copper(III). Ribas, X.*, Casitas, A.. 2, pp. 31-57 in “Ideas in Chemistry and Molecular Science. Where Chemistry Meets Life”, Ed.: B. Pignataro. Wiley-VCH, 2010. ISBN 978-3-5273-2541-2.