Main Content
Research Interests
One of the most extensive networks in eukaryotes for cofactor synthesis and trafficking is Fe/S protein biogenesis, which entails several cofactor trafficking principles (Figure 1). We are generally very interested in understanding the mechanisms of such metal trafficking reactions and the means in which a cell uses to maintain metal and cofactor specificity.
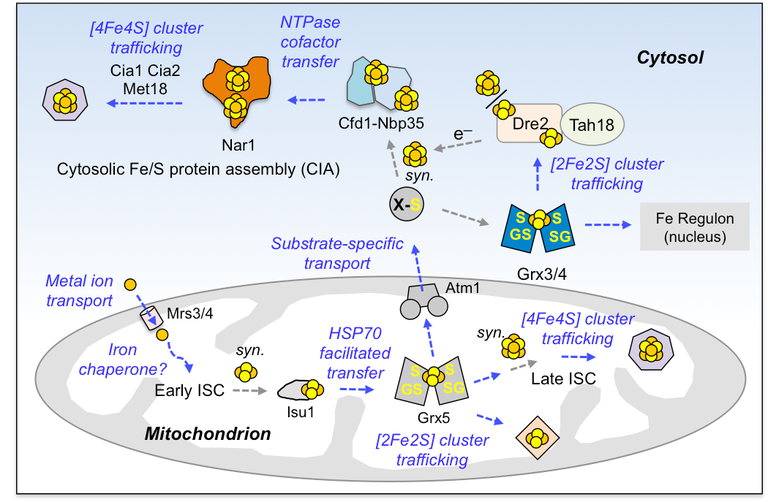
Figure 1: General cofactor trafficking and insertion principles exemplified by yeast Fe/S protein assembly machineries. The mitochondrial ISC system is the source for all cellular Fe/S clusters. The ISC is connected to the cytosolic CIA pathway via the transporter Atm1. A variety of protein specific trafficking and insertion reactions are required (blue text/arrows). syn, synthesis; ISC: iron-sulfur cluster assembly
From mononuclear metal ions like Zn, Cu, Fe, Ni, and Mo to more complex bioinorganic complexes like heme, Fe/S clusters, binuclear FeFe/NiFe clusters, and the molybdenum cofactor, a common theme in prokaryotic and eukaryotic cell biology is the use of cofactor specific trafficking pathways for the dedicated maturation of metalloproteins and metalloenzymes. Mononuclear transition metals are examples of cofactors whose cellular concentrations need to be tightly regulated, i.e., metal homeostasis, in order to prevent mis-metallation reactions, which can lead to the inactivation of enzymes. Whereas specific metallochaperones are known for trafficking Cu and Ni, Zn and Fe are far less defined as to how they reach their target proteins. Adding a layer of complexity to metal ion homeostasis is the need to traffic multi-nuclear metal centers after they have been synthesized in the cell.
Specific types of protein-based trafficking machineries typically exist for all transition metals and associated metal clusters. Six different principles can be differentiated, which are employed by these machineries for cofactor trafficking and insertion reactions.
-
Principle 1: is the use of substrate specific transporters, which are dedicated to carrying cargo through biological membranes
-
Principle 2: entails general cofactor trafficking proteins that pass cofactors off to further downstream proteins
-
Principle 3: is a specialized version of 2 in which energy from nucleotide triphosphate (NTP) hydrolysis is potentially used to control transfer reactions
-
Principle 4: is yet a further specialized version of 2 and 3: heat shock protein chaperones are also known to facilitate the transfer of clusters to downstream targets via ATP hydrolysis.
-
Principle 5: a further specialized variant of strategy 2, cofactor specific chaperones or insertases insert fully matured cofactors into target apoproteins
-
Principle 6: cofactors may also simply diffuse to their target proteins
Funded Research Project
In our SPP 1927 Fe/S for Life project, we focus on the cytosolic iron-sulfur assembly (CIA) component Nar1 (Figure 1). We will use biochemical methods (i.e., metal content analysis, protein-protein interactions), yeast cell biology, and biophysical methods to understand how Nar1 participates in Fe/S cluster trafficking.

Past Research Project
Marie Curie Fellowship 2015-2017

Project Titel: FourFeFourS