Main Content
Phosphazenyl phosphines PAP: The most electron rich uncharged phosphorus Brønsted and Lewis bases
It was discovered that phosphazenyl phosphines (PAPs) can be stronger P‐superbases than the corresponding Schwesinger type phosphazene N‐superbases. A simple synthetic access to this class of PR3 derivatives including their homologization is described. XRD structures, proton affinities (PA), and gas‐phase basicities (GB) as well as calculated and experimental pK values in THF are presented.
values in THF are presented.
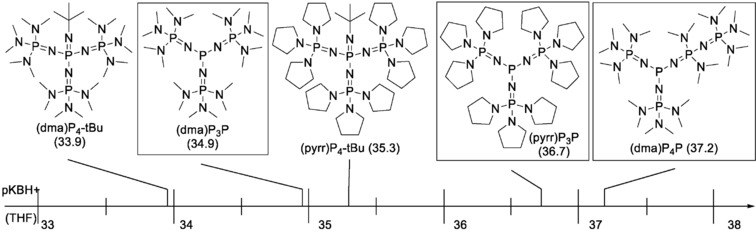
In contrast to their N‐basic counterparts, PAPs are also privileged ligands in transition metal chemistry. In fact, they are currently the strongest uncharged P‐donors known, exceeding classical and more recently discovered ligands such as PtBu3 and imidazolin‐2‐ylidenaminophosphines (IAPs) with respect to their low Tolman electronic parameters (TEPs) and large cone angles.
Publication: Phosphazenyl phosphines PAP: The most electron rich uncharged phosphorus Brønsted and Lewis bases.
S. Ullrich, B. Kovačević, X. Xie, J. Sundermeyer, Angew. Chem. Int. Ed. 2019, 58, 10335–10339; Angew. Chem. 2019, 131, 10443–10447. DOI: 10.1002/anie.201903342; DOI: 10.1002/ange.201903342