Main Content
Energy storage material
Various aspects of material science for energy storage materials are studied in the Weitzel group.
The CAIT technique is capable of providing detailed information on partial transport coefficients for ion and/or electron transport in battery materials.
In a second project, we aim at a holistic thermodynamic description of battery electrode materials. This is based on a Born cycle taking into account electronic and ionic contribution to the open circuit voltage of a lithium ion battery. As a first example, we have studied the LiCoO2 cathode material in the context of a Li//LiCoO2 battery [1].
The release of Li+ from stoichiometric LiCoO2 (LCO) – a typical battery electrode material – has been investigated by means of thermionic emission. Analysis of the data has led to an ionic work function of w(Li+)(LCO) = 4.1 eV. Combination of this value with the electronic work function w(e−) (LCO) = 5.1 eV, also measured in this work by photoelectron spectroscopy, and with information available from the literature allows setting-up a complete thermodynamic cycle for a Li//LiCoO2 battery (c.f. Figure 1). From this cycle an open circuit cell voltage of 2.4 eV has been derived in line with available literature information. This proof-of-principle study suggests that it is important to take into account ionic contributions to the OCV of a battery.
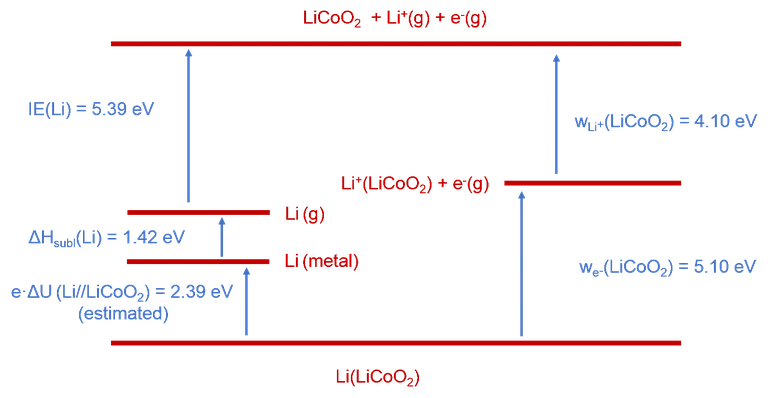
Figure 1. Born-type cycle of the energy balance in the Li // LiCoO2 system
In more recent investigations, we have demonstrated the ability to determine the ionic and the electronic work functions of lithium phosphate in an all-thermionic experiment [2].
Current efforts aim at determining the electronic and the ionic work function of lithium iron phosphate, Li(1-x)FePO4, as a function of the state of lithiation, x, both by experiment and by theory [3].
[1] Stephan Schuld, René Hausbrand, Mathias Fingerle, Wolfram Jaegermann, and Karl-Michael Weitzel,
Experimental Studies on Work Functions of Li+ Ions and Electrons in the Battery Electrode Material LiCoO2: A Thermodynamic Cycle Combining Ionic and Electronic Structure,
Advanced Energy Materials, 8, 1703411, (2018), (https://doi.org/10.1002/aenm.201703411 )
[2] Johanna Schepp, Dominik Plamper, Jon Both, Karl-Michael Weitzel, J. Appl. Phys. 128, 115108 (2020)
[3] Weitzel and Adams group, in preparation