Main Content
Femtosecond laser ion mass spectrometry
Ultra-high spectral resolution of laser radiation (labjargon: mono-chromatic) requires rather long laser pulses in the time domain or even cw laser radiation. In the other limit, ultra-short laser pulses in the time domain require a very broad spectrum. This is a manifestation of Heisenbergs’s principle. Due to the high peak power of femtosecond laser pulses, the interaction of these fs-pulses with molecules easily leads to ionization of these molecules, in fact in general even dissociative ionization. If coupled with a mass spectrometer - in our case a time of flight mass spectrometer - this constitutes femtosecond laser ionization mass spectrometry (fs-LIMS)
Fs-LIMS is interesting for several reasons. First of all, it is possible to ionize every single molecule in a laser focus. It is also possible to distinguish one from two molecules by counting devices. This makes fs-LIMS interesting for trace analysis. The intriguing fact is, that fs-LIMS is not only extremely sensitive, it can also be extremely specific.
E.g., we have discovered that fs-LIMS can be isomer specific, in particular if the fs-laser pulses are appropriately shaped, e.g. by an LCD device. Fs-LIMS thus provides a valuable tool to distinguish isomers, which may be difficult to distinguish by conventional MS techniques. Details for such studies are presented at https://www.uni-marburg.de/en/fb15/researchgroups/ag-weitzel/research/femtochemistry-of-ions/chemical-analysis-by-fs-lims
In a second application we employ fs-LIMS for the distinction of enantiomers, i.e. for chirality analysis. To this end, the fs laser pulses are circular polarized. Details of such research is presented at https://www.uni-marburg.de/en/fb15/researchgroups/ag-weitzel/research/femtochemistry-of-ions/analysis-of-chirality-by-femtosecond-laser-ionization-mass-spectrometry
An important tool in our fs-LIMS studies is the manipulation of the spectral phase of the laser pulses by means of a so-called spatial light modulator, essentially a LCD mask. The spectral phase of a laser pulse is given by
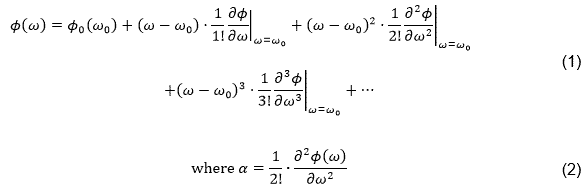
is the linear chirp parameter in the second order spectral phase coefficient.
If α=0 (and all higher order coefficients also), the pulse is termed transform limited. This leads to the shortest pulse for a given spectrum. From a chemical point of view, the transform-limited pulse is not the most interesting one. Neither does it provide the highest chemical sensitivity (see above) nor does it even provide the highest ionization yields - in contrast to conventional believe. In Fig. 1 we show ionization yields for the fs-laser ionization of ethane as a function of the linear chirp parameter alpha. Clearly, the highest ionization yields are observed for strongly negative linear chirp [1].
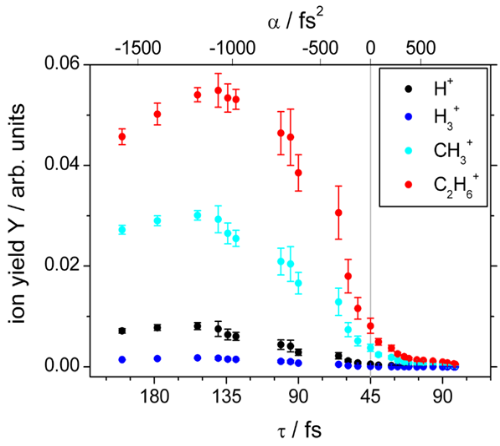
Fig. 1 Ion yields Y of the parent ion (C2H6+), as well as the fragment ions CH3+, H3+ and H+ from ethane as a function of the pulse length (FWHM, t) and the linear chirp parameter a for a pulse energy of 15 µJ. Taken from [1].
Literatur:
[1] Nora Schirmel, Nicola Reusch, Philipp Horsch and Karl-Michael Weitzel, Faraday Discussions, 163, 461, (2013)
Formation of fragment ions (H+, H3+, CH3+) from ethane in intense femtosecond laser fields – from understanding to control