Main Content
Formation of H3+ ions in intense femtosecond laser fields
Due to the high peak power of femtosecond laser pulses the interaction of these fs-pulses with molecules easily leads to ionization of these molecules, in fact in general even dissociative ionization. If coupled with a mass spectrometer - in our case a time of flight mass spectrometer - this constitutes femtosecond laser ionization mass spectrometry (fs-LIMS)
The interaction of fs-laser pulses with molecules is not only isomer-specific but also enantiomer-specific. However, it also allows to study the breaking and the making of chemical bonds in great detail.
The most intriguing case of breaking three bonds (C-H bonds) and making three bonds (H-H bonds) is involved in the formation of H3+ ions observed in the fs laser ionization of many hydrocarbon molecules [1]. A prominent example for formation of H3+ ions is the fs-laser ionization of ethane, for which we were – to the best of our knowledge – the first to describe a complete reaction path leading from an intermediate ethane dication all the way to the formation of H3+ (see Fig. 1) [2].
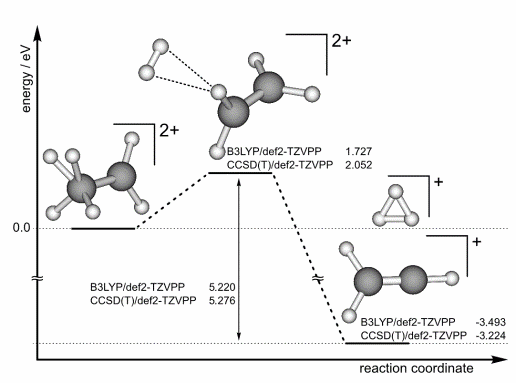
Fig.1 Reaction mechanism of the dissociation pathway of the ethane dication to H3+ and C2H3+ including the relevant energies.Later we have confirmed this reaction pathway by performing coincidence experiments between the H3+ and the C2H3+ ions [3].
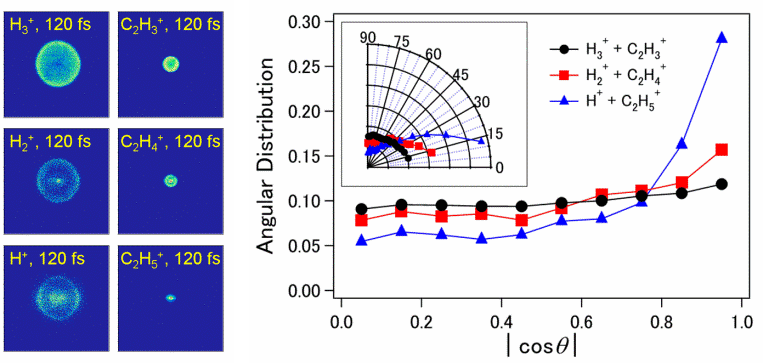
Fig. 2 Coincidence map confirming the correlation of H3+ and C2H3+ formation from ethane [3].
Finally we demonstrated that the formation of H3+ and of other fragments in the fs-LIMS of ethane can be controlled by tailoring the fs-laser field, in particular by controlling the linear chirp of the laser field [4],[5].
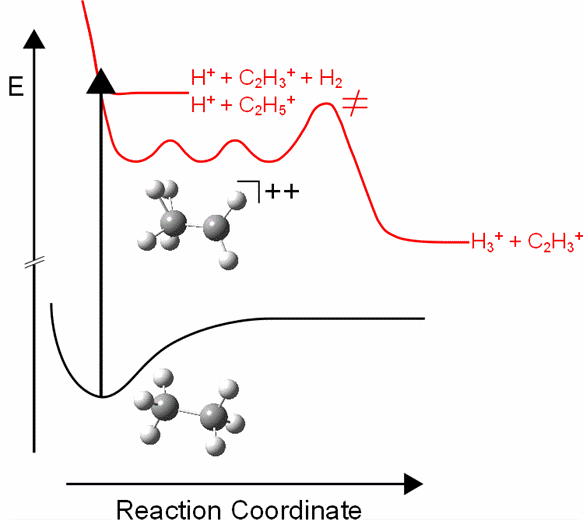
Fig.3 Schematic illustration of the potential energy curves leading from the equilibrium conformation of the neutral ethane (lower curve) to the formation of H+ and H3+ on the dicationic state. The characteristics of the potential energy curve for the H3+ formation illustrates the corresponding hydrogen scrambling and the transition states involved [5].
Literature
[1] K. Hoshina, Y. Furukawa, T. Okino, and K. Yamanouchi,
J. Chem. Phys., 129, 104302–6 (2008).
[2] P. Kraus, M.C. Schwarzer, N. Schirmel, G. Urbasch, G. Frenking, K.-M. Weitzel, J. Chem. Phys., 134, 114302 (2011)
Unusual mechanism for H3+ formation from ethane as obtained by femtosecond laser pulse ionization and quantum chemical calculations
http://dx.doi.org/10.1063/1.3561311
[3] R. Kanya, T. Kudou, N. Schirmel, S. Miura, K.-M. Weitzel, K. Hoshina, K. Yamanouchi
Hydrogen scrambling in ethane induced by intense laser fields: statistical analysis of coincidence events
J. Chem. Phys. 136, 204309 (2012);
http://dx.doi.org/10.1063/1.4720503
[4] N. Schirmel, N. Reusch, P. Horsch, and K.-M. Weitzel
The formation of fragment ions (H+, H3+, CH3+) from ethane in intense femtosecond laser fields – from understanding to control
Faraday Discussion, 163, 461-474, (2013)
http://dx.doi.org/10.1039/C3FD20152J
[5] N. Reusch, N. Schirmel, and K.-M. Weitzel,
Hydrogen migration in intense laser fields – analysis and control in concert,
in “Progress in Ultrafast Intense Laser Science”, Vol. 11, Springer Series in Chemical Physics 109, Eds: Yamanouchi, Nam and Martin, p. 1-21, (2014)