Main Content
State selective ion-molecule reactions

This project deals with the study of ion-molecule reactions. One of the central goals of this project is the investigation of ion-molecule reactions of state selected molecular ions. The term state-selection implies the knowledge of the electronic, vibrational and rotational state, in some cases even the parity. From the scientific point of view we are interested in the possibility of switching chemical reactions on and off by choice of the rotational state of the ion. This appears promising for small molecular ions, in which rather high rotational energy of up to 0.1 eV can be deposited by preparing the appropriate quantum state. From the technical point of view this is relevant in order to determine the optimum conditions for the application of plasma etching.
We are interested in ion-molecule reactions, which are relevant for plasma etching processes. This is the case for chemical processes involving either fluorinated hydrocarbons or hydrogen halide ions. (HX+, X=Cl, Br). In plasma etching not only the ion surface processes but also chemical reactions in the gas phase above the surface are very important. We are also interested in chemical reactions in interstellar space involving species like HCO+ etc.
Another project looks at the chemical interaction of alkali ions with bioorganic model compounds. The transport of alkali ions through ion channels is crucial for the transfer of signals in sensory perception. This transport involves many complex process involving diffusion but also chemical interaction.
Results from our work
We have studied the effects of reactant ion rotational excitation on the proton-transfer reactions of HBr+ and DBr+ with CO2 [1,2].
HBr+ / DBr+ + CO2 → HOCO+ / DOCO+ + Br
State selected HBr+ (DBr+) ions in the 2Π3/2(v+=0) and the 2Π1/2(v+=0) state were prepared by resonance enhanced multiphoton ionization (REMPI). This process, which results in ions with narrow rotational state distributions, was used to vary the mean rotational energy of the ions from 1 to 66 meV. A guided ion beam apparatus (see Fig. 1) was used to determine cross sections for collision energies Ec.m. in the center of mass system in the range of 0.23 to 1.90 eV. Ab initio calculations were performed to obtain energetic information about reactants, intermediates, and products (see Figure 2, [1]).
While the proton transfer is slightly endothermic for HBr+(2Π3/2) (PTendo), it is exothermic for HBr+(2Π1/2) (PTexo). This allows us to compare the cross sections for an endothermic and an exothermic reaction within the same reaction system [2]. In Fig. 3 the cross sections obtained for PTendo, and PTexo are shown as a function of the total energy Etot, which consists of contributions from the collision energy Ec.m., the mean rotational energy <Erot>, and, for PTexo, the spin-orbit energy Espin-orbit (Etot = Ec.m. + <Erot> + Espin-orbit). The data presented clearly occur in groups with a common Ec.m. but different <Erot>. It is obvious, that <Erot>, Ec.m., and Espin-orbit all have a different influence on the cross section. The results clearly cannot be explained by an effect of the total energy. While for PTendo decreasing Ec.m. and increasing <Erot> results in a smaller cross section, for PTexo the cross section decreases when both <Erot> and Ec.m. are increased. Note that, despite the differences, the highest cross section for one specific value of Ec.m. is always obtained for the smallest rotational energy considered, both for the endothermic and the exothermic reaction, at least well above the threshold of the reaction.
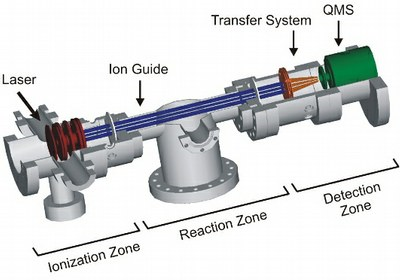
Fig. 1. Experimental setup [1].
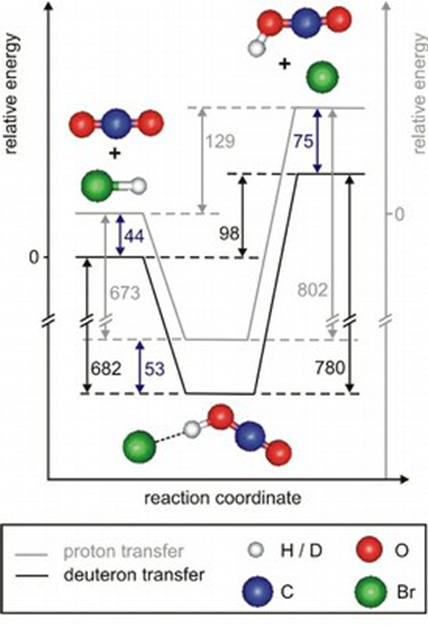
Fig.2. Energy profile for the proton-transfer reaction of HBr+ and CO2 and the deuteron-transfer reaction of DBr+ and CO2 with energetic differences in meV (based on ab initio calculations on PMP2/TZ2P level of theory) [1].

Fig. 3. Cross sections as a function of the total energy Etot = Ec.m. + <Erot> + Espin-orbit for the reactions of CO2 with HBr+(2Π1/2) (PTexo), and HBr+(2Π3/2) (PTendo). The inset shows a blow-up of the data points for the three lowest collision energies for PTexo. Cross sections measured for a common value of Ec.m. but different values of <Erot> are connected by a line in the inset for clarity [2].
More recently, the proton transfer dynamics in the HBr+ + CO2 reaction has also been studied by MD theory on an abinitio potential energy surface [3].
The self-reaction of state-selected HCl+ (DCl+) ions with HCl has been investigated in a guided ion beam setup. The absolute cross sections for proton transfer and deuteron transfer decrease with increasing center of mass collision energy, Ec.m.. The cross section for charge transfer (DCl+ + HCl) exhibits a maximum at Ec.m. = 0.5 eV. The cross section for PT and DT decrease significantly with increasing rotational angular momentum in the molecular ion, for the PT the cross section increases again for the highest angular momentum investigated. The rotational dependence of the cross section is rationalized by a simple model in which both the collision energy and part of the rotational energy are available for the reaction. The cross section exhibits a minimum under conditions where the rotational speed of the ion and of the neutral are identical. The contribution of the rotation to the total energy available itself depends on the collision energy [4]. Similar observations have been made for the self-reaction of HBr+ ions with HBr [5].
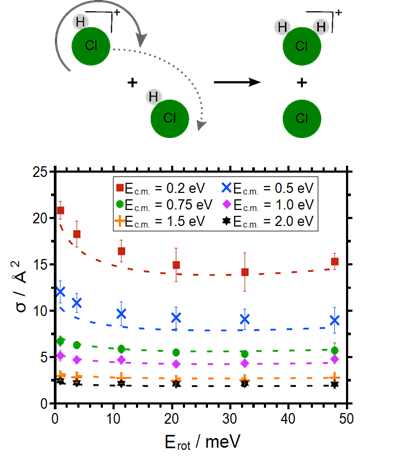
|
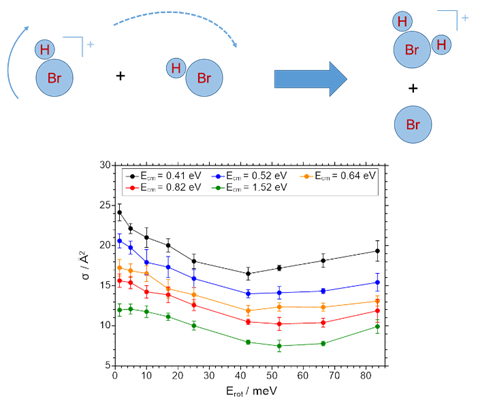
|
Fig. 4 Illustration of proton transfer reaction channels in the self reactions of HCl+ (left) and HBr+ (right).
Literature:
[1] L.Paetow, F.Unger, W.Beichel, G.Frenking, K.-M.Weitzel, J. Chem. Phys., 132, 174305, (2010)
Rotational dependence of the proton-transfer reaction HBr+ + CO2 → HOCO+ + Br :
I. Energy versus angular momentum effects
[2] L.Paetow, F.Unger, B.Beutel, K.-M.Weitzel, , J. Chem. Phys., 133, 234301, (2010)
Rotational dependence of the proton-transfer reaction HBr+ + CO2 → HOCO+ + Br :
II. Comparison of HBr+ (2Π3/2) and HBr+ (2Π1/2)
[3] Yuheng Luo, Kazuumi Fujioka, Alyson Shoji, William L. Hase, Karl-Michael Weitzel, and Rui Sun, J. Phys. Chem. A, 124, 9119−9127, (2020)
Theoretical Study of the Dynamics of the HBr+ + CO2 → HOCO+ + Br Reaction
[4] Till Uhlemann, Jens Wallauer, and Karl-Michael Weitzel
Self-reactions in the HCl+ (DCl+) + HCl system: a state-selective investigation of the role of rotation,
PCCP, 17, 16454-16461 (2015)
[5] Sebastian Schmidt, Dominik Plamper, Jasmin Jekkel, and Karl-Michael Weitzel, J. Phys. Chem. A, 124, 8461-8468, (2020)
Self-Reactions in the HBr+ (DBr+) + HBr System: A State-Selective Investigation of the Role of Rotation
Older work
[6] S. Athenstädt, F. Unger, K.-M. Weitzel, Z. Phys. Chem., 221, 571-584, (2007)
Rotational dependence of the proton transfer reaction HBr+ (N+) + CO2 → HOCO+ + Br
[7] M. Malow, K. Brembs and K.-M. Weitzel, Z. Phys. Chem., 215, 737-748, (2001)
Ion-molecule reactions of state selected HCl+ ions with carbondioxide and ethene.
[8] M. Michel, M.V. Korolkov, M. Malow, K. Brembs and K.-M. Weitzel, Phys. Chem. Chem. Phys., Vol. 3, (2001), 2253-2257
Unimolecular and bimolecular reactions of state selected HCl+ ions formed via the R(1) pump line of the f 3Δ2 ← 1Σ+ REMPI spectrum