Main Content
Transport of ions through ultra-thin deposited fims
Transport of ions through ultra-thin deposited films
The bombardment induced ion transport (BIIT) approach developed in our group not only allows to measure ionic conductivities of solid electrolytes, which are ionic conductors in the first place, but also of polymer films. Many polymer films are considered “electrically insulating”, which more precisely should read “electronically insulating”. We can , e.g., easily demonstrate that ultra-thin PPX (poly-para-xylylene) films are capable of transporting alkali ions.
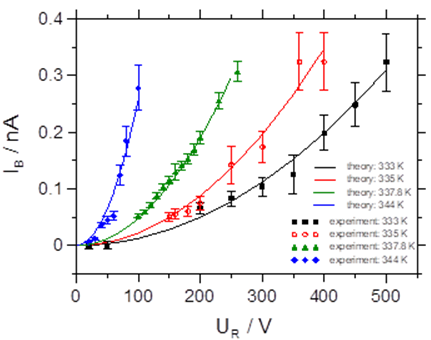
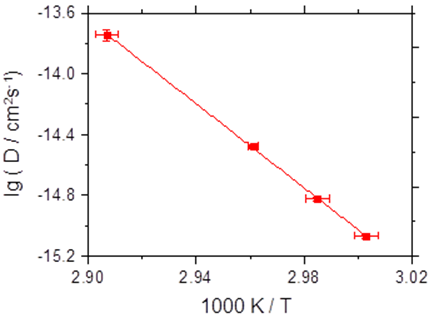
More specifically, we have reported on the first investigation of bombardment induced ion transport of potassium ions through a thin PPX film deposited on a metal electrode. We demonstrate that the current scales quadratically with the ion beam energy. Analysis of these current-voltage curves yields the diffusion coefficient. The measurement of the diffusion coefficient as a function of the film temperature yields the activation energy for potassium ion hopping through PPX-films to be 2.74 eV ± 0.18 eV [1].
|
|
Fig. 1 Left graph: current-voltage data for a thin PPX film as a function of the temperature. Right graph: Arrhenius plot of the diffusion coefficient of K+ in the PPX film.
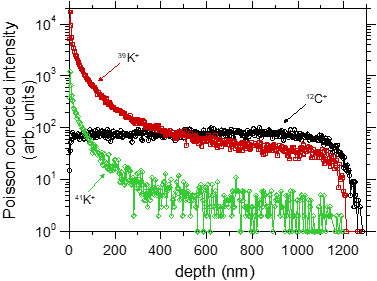
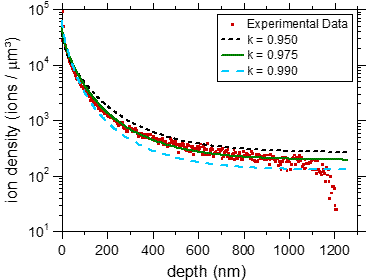
In a separate experiment we have demonstrated that BIIT of potassium ions in PPX leads to a concentration profile of potassium reaching through the entire film [2].
|
|
Fig. 2 Left graph: experimental concentration profiles measured via ToF-SIMS, The C+ signal reflects the bulk properties as well as the thickness of the film. The K+ profiles clearly reach through the entire film. Right graph: Experimental K+ profile (symbols) together with calculated profiles assuming concentration dependent diffusion coefficients. For further details see ref. [2]
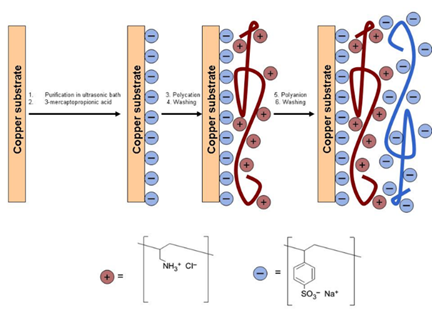
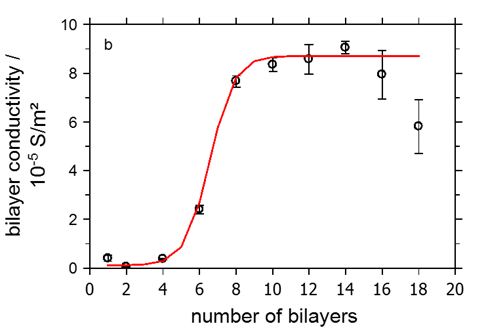
While polymer films do not contain free charge carriers prior to the CAIT experiment, there is another class of polymer materials “full of” charges, i.e. the poly-electrolyte membranes (PEMs). PEMs are in general prepared by layer-by-layer technique, where cationic and anionic layers alternate. The thinnest membranes (these are not bio membranes) is inherently a single bi-layer. We have measured the ionic conductivity of (PAH/PSS)n membranes as a function of the number of bi-layers, n. Our data published include the conductivity of a single bi-layer with a thickness on the order of 4nm [3].
|
|
Fig. 3 Left graph: schematic representation of the layer-by-layer technique. Right graph: bilayer conductivity vs. number of bilayers. The solid line is the result of a model calculation.
[1] Susanne Schulze, Martin Schäfer, Andreas Greiner and Karl-Michael Weitzel
Bombardment induced ion transport – Part III: Experimental potassium ion conductivities in poly(para-xylylene)
PCCP, 15, 1481-1487, (2013)
[2] Susanne Schulze, Julia Zakel, Martin Schäfer and Karl-Michael Weitzel
Potassium ion transport through poly-para-xylylene films
IEEE-TDEI, 19, 1167-1174 (2012)
[3] Veronika Wesp, Matthias Hermann, Martin Schäfer, Jonas Hühn, Wolfgang J. Parak, Karl-Michael Weitzel
Phys.Chem.Chem.Phys., 18, 4345, (2016)