Main Content
p53 mutations and chemo(radio)resistance of head and neck squamous cell carcinomas (HNSCCs)
Several studies described the central role of the tumor suppressor protein p53 in therapy response of HNSCC and other cancers. For more than 20 years it is known that the nuclear localization of p53 is required for its proper function emphasizing its main role as a transcription factor. Cancers in the head and neck area are mainly (>90%) squamous cell carcinomas (HNSCCs), which in more than 70% carry mutations in the TP53 gene. In the remaining 20-30% of HNSCC cases carrying wild type p53, the tumor suppressor is also inactivated, which is considered to be mainly due to a concomitant infection with high-risk human papilloma viruses (HPVs) particularly the type 16. Initial studies with HNSCC cell lines revealed a substantial number of TP53 mutations affecting the C-terminus of p53 resulting in loss of the C-terminal nuclear localization signal (NLS) (Figure 1).
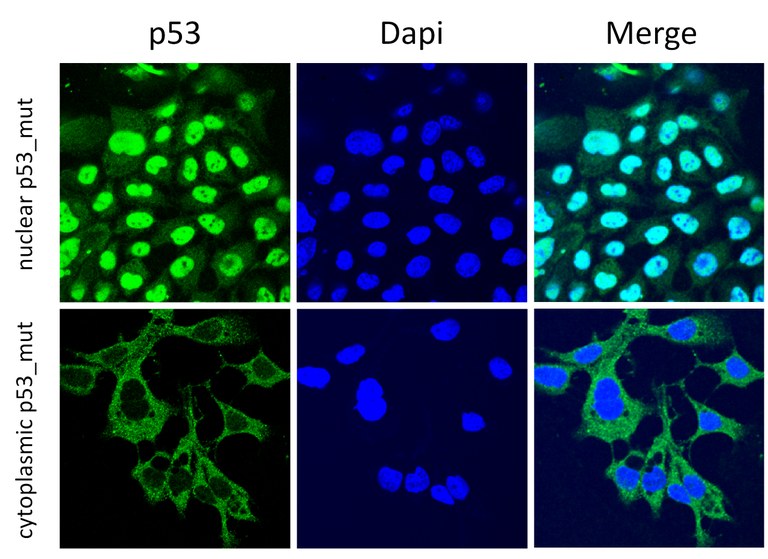
Figure 1. Depicted are confocal images from two HNSCC cell lines, both carrying TP53 mutations. The cell line shown in the upper panel carries a typical hot spot mutation in the DNA binding domain of p53 not affecting the C-terminal NLS of the protein, demonstrating a dominant nuclear accumulation of p53_mut. In sharp contrast, the other cell line (lower panel) carries a premature stop codon, resulting in a C-terminally truncated p53 protein with loss of the C-terminal NLS and cytoplasmic sequestration.
Comparing HNSCC cell lines with nuclear or cytoplasmic p53_mut regarding their sensitivity to cisplatin (CDDP), the major chemotherapeutic agent used in the treatment of HNSCC, revealed a significantly lower CDDP sensitivity of HNSCC cell lines with cytoplasmic p53_mut (Figure 2).
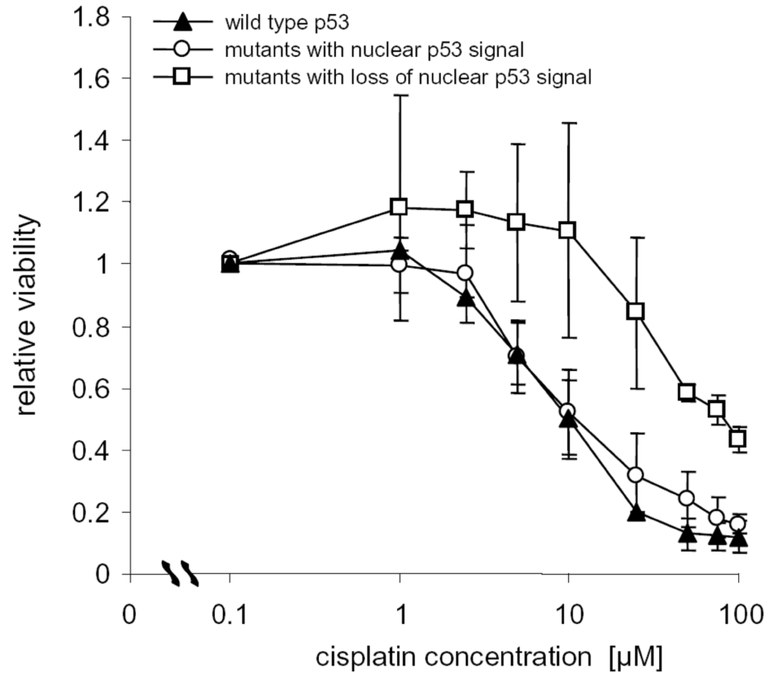
Figure 2. The viability of HNSCC cells with cytoplasmic p53_mut is significantly less affected after CDDP treatment. In particular, at therapeutic concentrations (1-10 µM) of CDDP, the viability of HNSCC cells with nuclear p53_mut dropped dramatically, whereas no reduction in viability is seen for cells with cytoplasmic p53_mut.
The gene expression profile of HNSCC cells with cytoplasmic and nuclear p53_mut was determined, to identify potential candidate genes involved in CDDP sensitivity. Some of the most differentially regulated candidate genes belong to the ATP-binding cassette (ABC) transporter family, known to be involved in transport of drugs and xenobiotica such as chemotherapeutic agents like CDDP out of the cell (Figure 3).
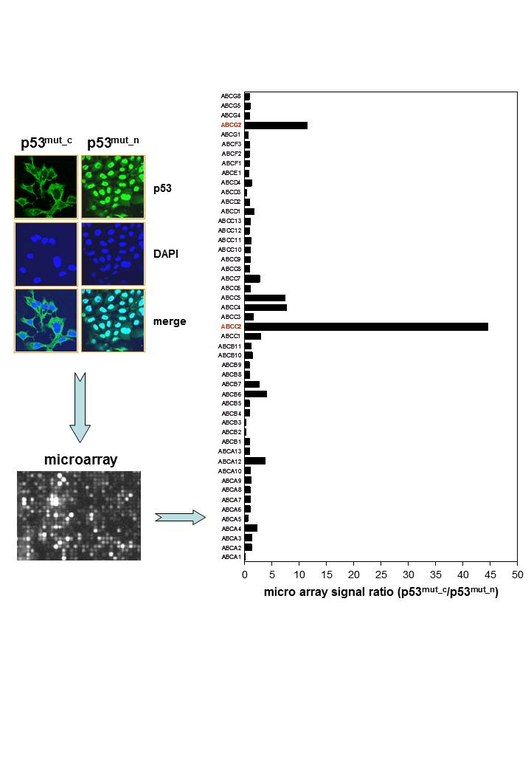
Figure 3. The ABC Transporters ABCC2 and ABCG2 are upregulated in HNSCC cells with cytoplasmic p53_mut.
A subsequent inhibition of ABCC2 and ABCG2 transporters could render HNSCC cells, including resistant tumor cells with cytoplasmic p53_mut, more sensitive to CDDP treatment (Figure 4).
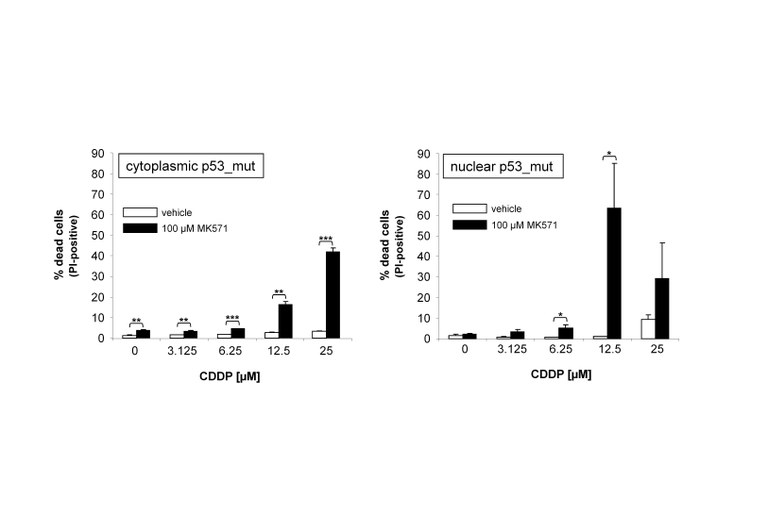
Figure 4. Treatment of HNSCC cells with MK571, which inhibits ABCC2 and ABCG2, sensitizes tumor cells to cisplatin.
Furthermore, resistant HNSCC cells with cytoplasmic p53_mut exhibit significantly higher levels of reduced glutathione (GSH), which is required to fend off cytotoxic effects such as those caused by CDDP treatment but also for proper ABC transporter mediated efflux of toxic compounds such as CDDP. All these factors are promoting CDDP resistance (Figure 5).
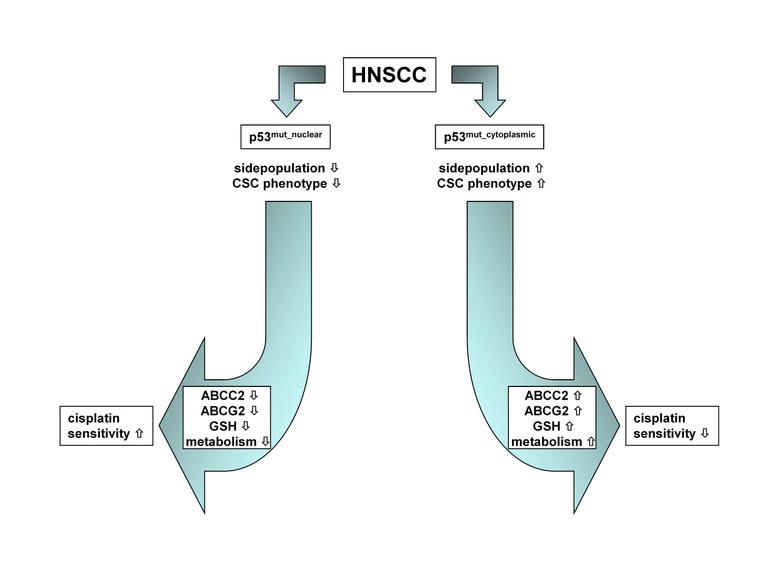
Figure 5. Model depicting the possible interrelationship between the nucleocytoplasmic distribution of p53_mut, ABC transporter expression, cellular GSH levels and CDDP sensitivity.
The goal of current investigations is to evaluate if influencing the GSH/GSSG system with clinically approved GSH inhibitors such as the GSH-S-transferase inhibitor sulfasalazine, could improve chemo(radio) sensitivity of resistant HNSCC tumors.