Main Content
Crystal Growth
Here we present some methods we use to grow single crystals of organic substances.
Solution growth technique
There are two possibilities to crystallize a substance from solution. The first approach consists in producing a solution at higher temperatures and cooling it down slowly afterwards. For example, you can use a solvent that has a boiling point above 100°C and heat it up to 100 °C in a water bath. If the material you want to crystallize is entirely dissolved, the heating can be turned off in order to slowly cool down the water and thereby the solution. If the solution reaches a temperature at which it is in a state of supersaturation, the material starts to crystallize. If you slowly surpass the so-called Ostwald-Miers area, well-defined crystals can be cultivated. As an example you can see here a perylene crystal, which has been grown by this method from toluene.
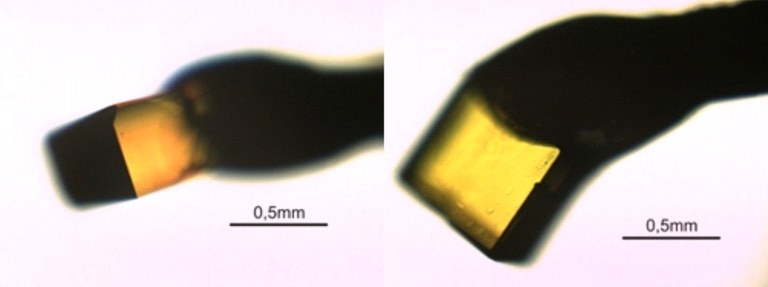
Figure 1: α-perylene grown from cooled-down toluene, shown from different perspectives; note the pleochroism.
Alternatively, you can prepare a heatable bath of silicone oil to ensure that temperature fluctuations can be kept down to a minimum. If you are limited to a volatile solvent, the use of a Dimroth Condenser is recommended, in which concentration fluctuations can occur. Vibrations can influence this process in a negative way, too. In order to avoid this the whole system can, for example, be put under a vibration-damped cover.
There is one further method: You can prepare a solution at room temperature and then let the solvent slowly evaporate. The speed of the evaporation can essentially be determined by the aperture of the container. In some cases it can be useful to grow crystals this way. For example, perylene develops in another polymorphism if the solvent is evaporated and not cooled down.
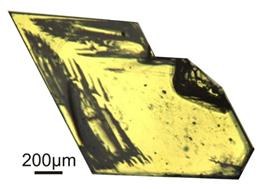
Figure 2: β-perylene grown from evaporated toluene solution.
From gas phase
a) Hot Wall Deposition
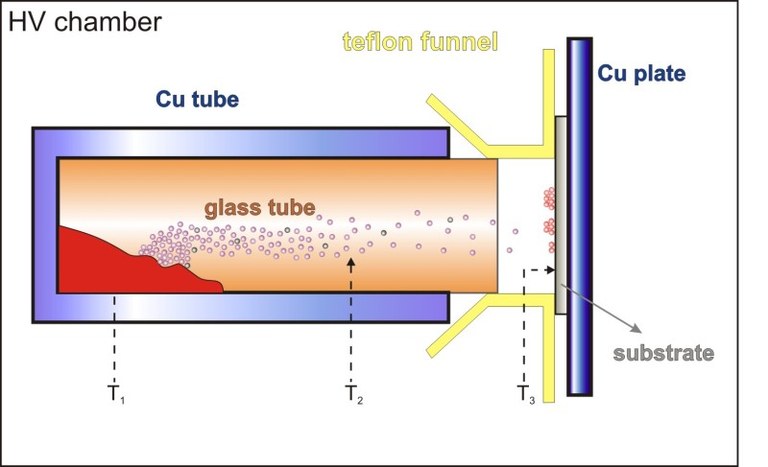
Figure 3: Schematic diagram of the Hot Wall Deposition.
The so-called Hot Wall Deposition is performed in a UHV chamber, in which a glass tube and the substrate are connected via a teflon ring. In the tube, an atmosphere of the material to be crystallized is generated. The temperatures of the tube and the substrate have to be optimized by trial and error (see figure 3). In this way it is possible to grow thermodynamically stable microcrystals.
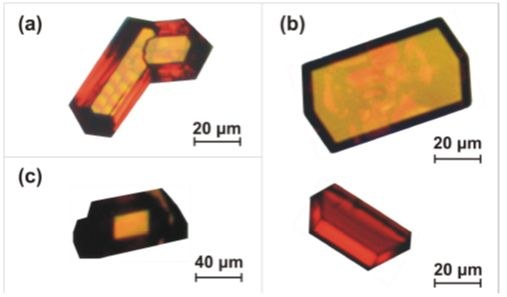
Figure 4: Rubrene crystals obtained by Hot Wall Deposition.
b) Gradient Sublimation
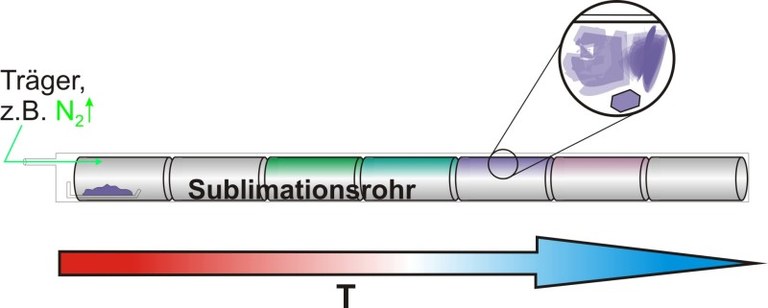
Figure 5: Schematic diagram of a sublimation tube.
By means of this method a material is evaporated in a long glass tube and transported with the help of an inert carrier gas to cooler areas where the crystallization takes place subsequently. Although this method is mainly used for purification purposes, as contaminations crystallize in different areas than the actual material due to the temperature gradient in the tube, this principle is also used to grow crystals selectively.
c) OMBD in thin liquid films
Liquids with a very low vapor pressure can also be brought into a UHV chamber. If a substrate is coated with a thin film of silicon oil (alternatively also ionic liquids) and the original material is steamed into the film by the use of a Knudsen cell, single crystals of large lateral dimensions can be grown under favorable conditions.
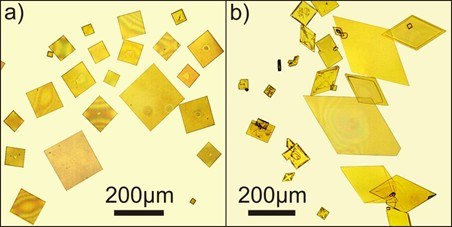
Figure 6: Both phases of perylene (see above), grown in a thin film of silicon oil.