Main Content
Substrate Preparation
The investigation of organic thin films requires careful attention to the choice of substrate material. The order and the structure of a substrate surface can affect characteristics of adsorbed materials. Common types of solid surfaces used in experiments are:
1. Single crystalline: metals, metal oxides...

Although many technical devices are based on the usage of polycrystalline surfaces due to their low cost and the ease of production only single crystalline surfaces are suitable for studying microscopic processes of a surface on a well-defined basis.
In experiments we deal with different crystallographic surface orientations of single crystals (metals, metal oxides). Different surface orientations are achieved by cleavages along crystallographic planes of the bulk structure.
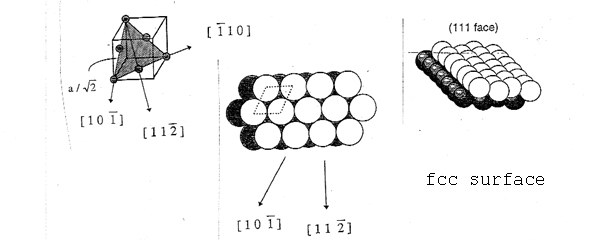
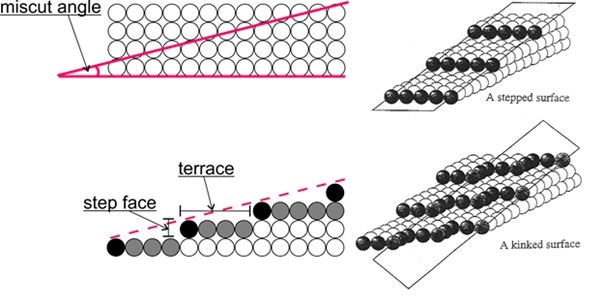
Important for the application are vicinal surfaces – stepped surfaces of single crystals obtained by cutting at a relatively small angle (miscut angle) to one of the low-index surfaces. This results in a surface consisting of terraces with an atomic arrangement identical to the corresponding low index surface, separated by monoatomic steps. This allows to mimic the influence of defects (like step edges) in an experiment.
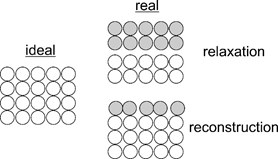
Real surfaces though may differ from the ideal cut of a bulk crystal. Ideal symmetry of a surface can be broken due to:
a) surface relaxation and reconstruction
b) the presence of unbalanced bonding in the topmost layer
2. Polycrystalline (most technical surfaces): metals, powder...

3. Amorphous (lacks long-range order): SiO2, polymer...

Amorphous surfaces are isotropic, hence, adsorbed layers do not exhibit a preferred growth orientation, which can be demonstrated in XRD measurements. As a consequence, they induce the formation of the free solid (“bulk”)-like structure. Some molecules exhibit a changed structure in the percent range for thin films with thicknesses below 100 nm, which is commonly called “thin film phase”.
A well-established amorphous surfaces is silicon dioxide (SiO2) which forms as a native oxide layer on the surface of commercially available, single crystalline silicon wafers. It is atomically smooth in an area of many micrometers.
Here are some examples of substrates we typically use in our experiments:
▪ Metals: Commonly used are group 11 elements (Cu, Au, Ag, also known as coinage metals). Their high surface binding energy induces strong molecule-substrate bonding in a first monolayer. It is more strongly bound than higher layers. By intentionally induced thermal desorption of the upper layers a well-defined single layer of molecules can be prepared, which in turn can be utilized as contact primer for subsequent adsorbates. The same is true for self-assembling monolayers (SAMs), whose anchor groups adhere very well to the coinage metal.

The preparation of atomically smooth metal surfaces with long range order is a long-lasting process. This process can be facilitated by depositing some 100 nanometers of the desired metal on the sheet silicate “Mica” via physical vapor deposition (PVD). Highly ordered metal layers in (111)-orientation are formed. Before their usage in experiments the metal films undergo cycles of ionic sputtering and heating in vacuum to achieve a sufficient level of surface cleanliness and smoothness. All in all, preparation and cleaning is less time-consuming than for metal single crystals.
▪ Metal oxides: Single crystals (TiO2, ZnO) are transparent conductive oxides and interesting for their promising applications in photovoltaic and photocatalysis. Substrate transparency enables optical and spectroscopic analysis of adsorbed layers. In experiments we also deal with poly crystalline (powder) and amorphous (SiO2) metal oxides.

▪ Alkali halides: Inorganic salts, such as KCl and NaBr, offer a highly ordered surface and transparency at the same time. Many molecular adsorbates display a preferred growth direction with respect to the atomic structure of the salt surface, the salt thus works as a template for deposited organic molecules and represents an interesting model system for characterization of growth phenomena. Samples can be prepared by splitting off slices from commercially available single crystalline columns.