Main Content
Thermal Desorption Spectroscopy (TDS)
Molecules bound to a surface with a certain energy can get released from it by increasing the temperature. In TDS, the entirely or partially desorbing molecules are detected by means of a mass spectrometer (QMS) while the surface is heated with a defined rate. This allows to draw conclusions regarding the thermal stability of the molecular species.
In our research TDS is used to characterize organic thin films which are evaporated on different substrates or formed by immersion. The temperature necessary to release molecules from the surface is a direct measure for the desorption energy. Thereby, the system must be capable to detect a low amount of molecules and the temperature over time has to be regulated very precisely. It requires a high and stable vacuum and an extremely sensitive measurement technique.
Typical questions addressed with this technique are the following:
- Is a molecule covalently bound to the surface or is it just physisorbed? Is it arranged in a single layer or are several layers present?
→ Detection of a characteristic mass and analysis of the desorption temperature and peak shape.

Figure 1: QMS Signal acquired for different film thicknesses of pentacene. Multi-layer desorption takes place at lower temperatures than that of the bottommost monolayer (blue) [1].
- Do different species join to form a stabilized heterostructure?
→ Comparison of single spectra with those obtained for the mixture of substances.
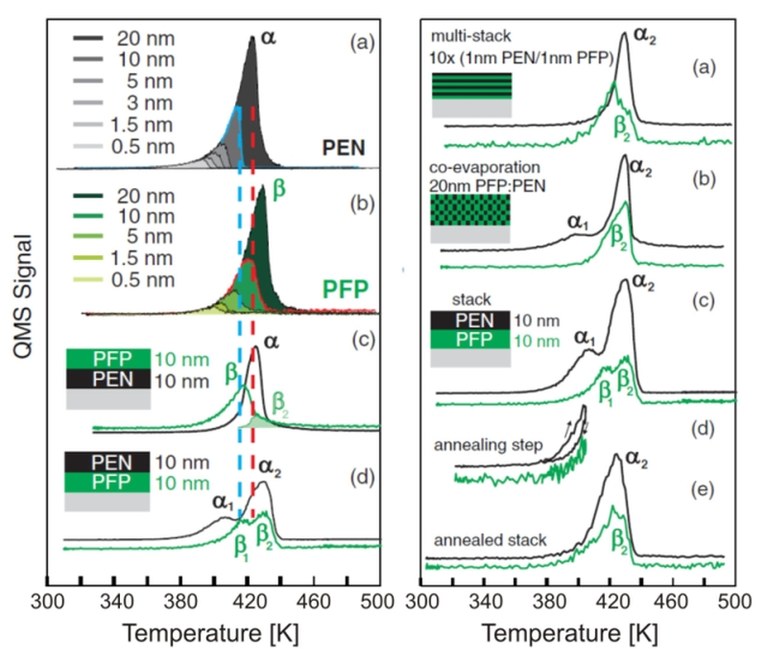
Figure 2: The thermal desorption spectrum for the PEN/PFP mixture shows characteristics that indicate the intermixing of the two substances to a heterostructure [2].
- How does a film change over time or upon active manipulation?
→ Comparison of the TD spectra with different masses at different times.

Figure 3: Here, electron irradiation activates a stable cross-linking of the molecules, thereby changing the structure of the desorbing fragments [3].
In our lab OMBD apparatuses I und II („Molly“) are used to perform TDS measurements. Accessible are masses from 0 to 300 u/e in a temperature range from 150 K to above 850 K (with stable heating rate).
At the bottom you can see a view into the chamber: Well visible are the Feulner cup and a heated sample; the red-colored QMS body can be seen in the background.

Some exemplary publications of our group where thermal desorption spectroscopy has been utilized are:
- [1] Thermally activated dewetting of organic thin films: the case of pentacene on SiO2 and gold.
D. Käfer, C. Wöll, G. Witte
Appl. Phys. A 95 (1), 273-284 (2009)
Full Text - [2] Thermally activated intermixture in pentacene-perfluoropentacene heterostructures.
Tobias Breuer and Gregor Witte
J. Chem. Phys. 138 (11), 114901 (2013)
Full Text - [3] Molecular mechanisms of electron-induced cross-linking in aromatic SAMs.
A. Turchanin, D. Käfer, M. El-Desawy, Ch. Wöll, G. Witte, A. Gölzhäuser
Langmuir 25 (13), 7342-7352 (2009)
Full Text - Formation and Stability of Phenylphosphonic Acid Monolayers on ZnO: Comparison of in-situ and ex-situ SAM Preparation.
Alexandra Ostapenko, Tobias Klöffel, Bernd Meyer, and Gregor Witte
Langmuir 32 (20), 5029−5037 (2016) • DOI: 10.1021/acs.langmuir.6b00487
Full Text - Epitaxial TTF-TCNQ Thin Films on KCl(100): New Preparation Methods and Observation of Interface-Mediated Thin Film Polymorph.
Alexander Mänz, Tobias Breuer, and Gregor Witte
Crystal Growth & Design 15 (1), 395–403 (2015)
Full Text - Structural and optical properties of pentacene films grown on differently oriented ZnO surfaces.
M. El Helou, E. Lietke, J. Helzel, W. Heimbrodt, and Gregor Witte
Journal of Physics: Condensed Matter 24 (44), 445012 (2012)
Full Text