Main Content
X-ray Photoelectron Spectroscopy (XPS)
X-ray radiation exhibits photon energies in the range of keV. This energy is higher than the binding energy of core electrons. If such a photon is absorbed, the absorbing electron has sufficient energy available to leave the atom. If the energy of the incident photons is known, the kinetic energy of the electrons can be measured in order to derive the binding energy.
Since the binding energy is element-specific x-ray photoelectron spectroscopy can be used to analyze the elemental composition of the sample. As the mean free path of the photo electrons is furthermore in the range of a few nm the method is surface-sensitive. This effect can be used for example to check the cleanliness of surfaces.
As already mentioned core electrons are investigated by means of this method. For valence band electrons, UV light is used instead of x-ray radiation thus leading to the related UPS method. The advantage of investigating core electrons is that these are not involved in bonds and that their energy levels are therefore hardly affected by the binding situation of the atom. Having a closer look to the binding energies, nevertheless minor energy shifts can be observed depending on the chemical vicinity of the atom. This so-called "chemical shift" can be used to obtain information about the binding situation of the atoms.
Typical questions are:
- Is a surface clean on the microscopic level?
→ Search for carbon as a component of characteristic impurity
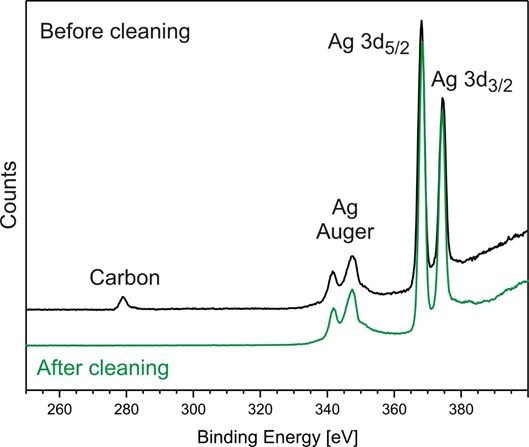
Figure 1: Excerpt from an XPS spectrum of a silver surface before and after purification from carbon
- What is the chemical composition of a layer? Do molecules bind to surfaces or are they physisorbed?
→ Investigation of "chemical shifts"
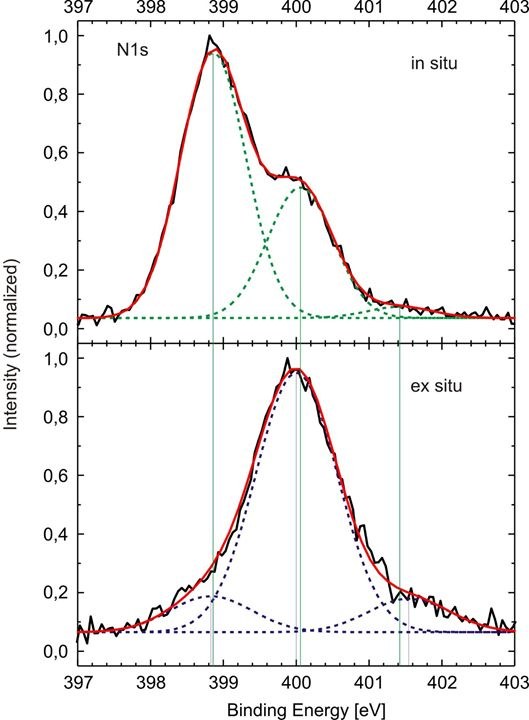
Figure 2: Left: Separation of the N1s signal indicating that nitrogen is present in different chemical surroundings.
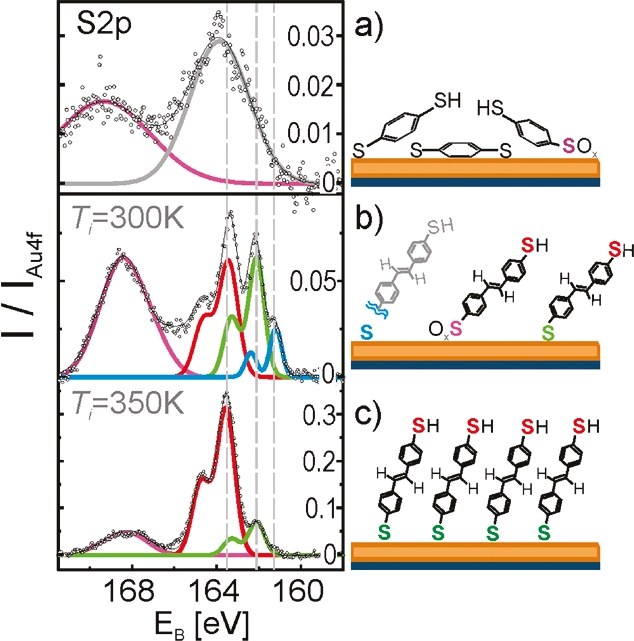
Figure 3: Similar spectra of sulfur. From the separation of the measured data in four different S2p doublets conclusions can be drawn about the ordering of the dithiol SAMs [1].
Some exemplary publications of our group where x-ray photoelectron spectroscopy has been utilized are:
- [1] Immobilization of quantum dots via conjugated self-assembled monolayers and their application as a light-controlled sensor for the detection of hydrogen peroxide.
W. Khalid, M. El Helou, T. Murböck, Z. Yue, J.-M. Montenegro, K. Schubert, G. Göbel, F. Lisdat, G. Witte and W. J. Parak
ACS Nano 5 (12), 9870-9876 (2011)
Full Text - Formation and Stability of Phenylphosphonic Acid Monolayers on ZnO: Comparison of in-situ and ex-situ SAM Preparation.
Alexandra Ostapenko, Tobias Klöffel, Bernd Meyer, and Gregor Witte
Langmuir 32 (20), 5029−5037 (2016) • DOI: 10.1021/acs.langmuir.6b00487
Full Text - Substrate induced thermal decomposition of perfluoro-pentacene thin films on the coinage metals.
Christian Schmidt, Tobias Breuer, Stefan Wippermann, Wolf Gero Schmidt, and Gregor Witte
Journal of Physical Chemistry C 116 (45), 24098-24106 (2012)
Full Text