Main Content
2013
Inhalt ausklappen Inhalt einklappen ACS NANO: "Cell-imprinted substrates direct the fate of stem cells"
Morteza Mahmoudi, S. Bonakdar, M. A. Shokrgozar, H. Aghaverdi, R. Hartmann, A. Pick, G. Witte, W. Parak
ACS Nano 7 (10), 8379-8384 (2013), DOI: 10.1021/nn403844q
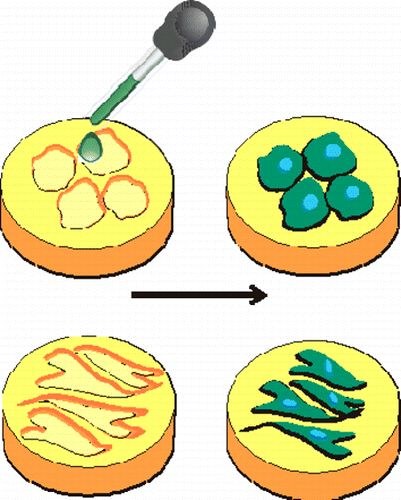
Smart nano-environments were obtained by cell-imprinted substrates based on mature and dedifferentiated chondrocytes as templates. Rabbit adipose derived mesenchymal stem cells (ADSCs) seeded on these cell-imprinted substrates were driven to adopt the specific shape (as determined in terms of cell morphology) and molecular characteristics (as determined in terms of gene expression) of the cell types which had been used as template for the cell-imprinting. This method might pave the way for a reliable, efficient, and cheap way of controlling stem cell differentiation. Data also suggest that besides residual cellular fragments, which are presented on the template surface, the imprinted topography of the templates plays a role in the differentiation of the stem cells.Inhalt ausklappen Inhalt einklappen ACS APPL. MATER. IN TERFACES: "Diffusion-controlled growth of molecular hetero-structures: fabrication of 2D, 1D and 0-Dimensional C60-nanostructures on pentacene substrates"
T. Breuer, G. Witte
ACS Applied Materials & Interfaces 9740-9745 (2013), DOI: 10.1021/am402868s
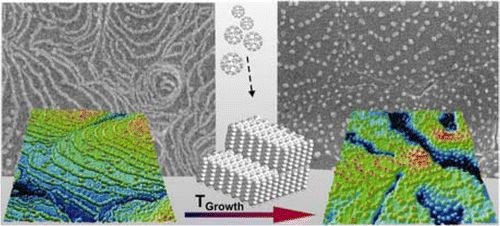
A variety of low dimensional C60 structures has been grown on supporting pentacene multilayers. By choice of substrate temperature during growth the effective diffusion length of evaporated fullerenes and their nucleation at terraces or step edges can be precisely controlled. AFM and SEM measurements show that this enables the fabrication of either 2D adlayers or solely 1D chains decorating substrate steps, while at elevated growth temperature continuous wetting of step edges is prohibited and instead the formation of separated C60 clusters pinned at the pentacene step edges occurs. Remarkably, all structures remain thermally stable at room temperature once they are formed. In addition the various fullerene structures have been overgrown by an additional pentacene capping layer. Utilizing the different probe depth of XRD and NEXAFS we found that no contiguous pentacene film is formed on the 2D C60 structure, whereas an encapsulation of the 1D and 0D structures with uniformly upright oriented pentacene is achieved, hence allowing the fabrication of low dimensional buried organic hetero-structures.Inhalt ausklappen Inhalt einklappen JCP: "Thermally activated intermixture in pentacene-perfluoropentacene heterostructures"
T. Breuer, G. Witte
Journal of Chemical Physics 138 (11), 114901 (2013), DOI: 10.1063/1.4795004
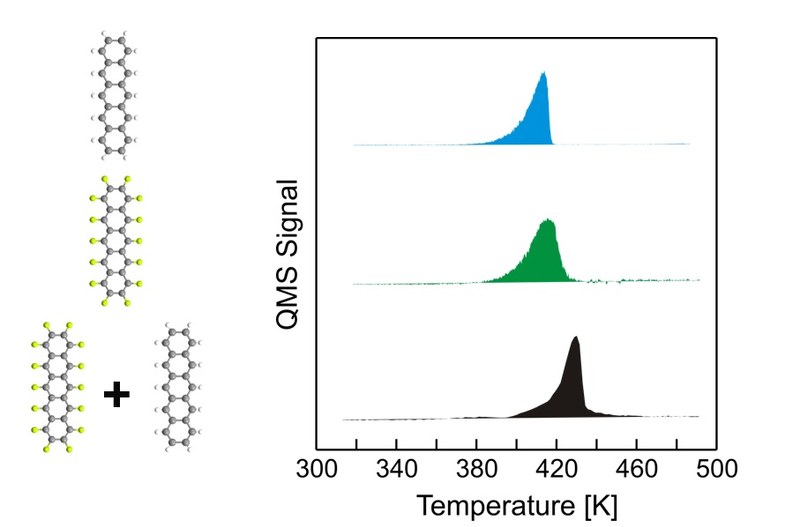
Using thermal desorption spectroscopy (TDS) the thermal stability of binary pentacene/perfluoropentacene (PEN/PFP) thin films has been investigated for various preparation protocols. Variation of stoichiometry ratio reveals a significantly enhanced thermal stability in comparison to the single compounds only for films with equimolar stoichiometry. The stabilization also depends on the preparation method and was found for co-deposition as well as for multistacks and subsequently grown PEN/PFP-stacks but not for stacks grown in the reversed order. By systemically varying the substrate temperature during deposition, we prove that the resulting intermixture is caused by a thermally activated diffusion during film growth and not due to post-deposition diffusion induced upon heating during TDS measurements. The different extents of thermal stabilization are discussed in the context of the film morphology studied by means of atomic force microscopy (AFM). For complementary information, optical absorption spectra of the heterostructures are analyzed, where the arisal of new absorption bands and the extinction of excitonic bands existing in the pure compounds are identified as decisive criteria to judge the efficiency of intermixture.Inhalt ausklappen Inhalt einklappen EurJOC: "Synthesis and solid-state structures of 6,13-Bis(trifluoromethyl)- and 6,13-Dialkoxypentacene"
J. Schwaben, N. Münster, T. Breuer, M. Klues, K. Harms, G. Witte, U. Koert
European Journal of Organic Chemistry 2013 (9), 1639-1643 (2013), DOI: 10.1002/ejoc.201201714
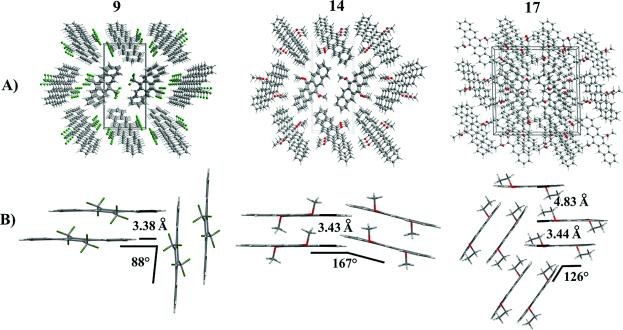
Novel 6,13-disubstituted pentacenes were synthesized. Their electrochemical and optical properties and their packing motif in the solid state cold been determined. Treatment of pentacenequinone with TMSCF3 (Ruppert’s reagent) and deprotection led to the 6,13-bis(trifluoromethyl)-pentacene-6,13-diol which was aromatized to 6,13-bis(trifluoromethyl) pentacene using PBr3. 6,13-Dialkoxypentacenes were accessible via alkylation of the corresponding hydroquinopentacenes using dialkyl sulfates. 6,13-Bis(trifluoromethyl) pentacene and 6,13-dimethoxypentacene exhibit slipped face to face π-stacking in the solid state while 6,13-diethoxypentacene forms pairs of π-stacking molecules in the solid state.