Main Content
2016
Inhalt ausklappen Inhalt einklappen PHYS. CHEM. CHEM. PHYS.: "Self-assembly of partially fluorinated hexabenzocoronene derivatives in the solid state"
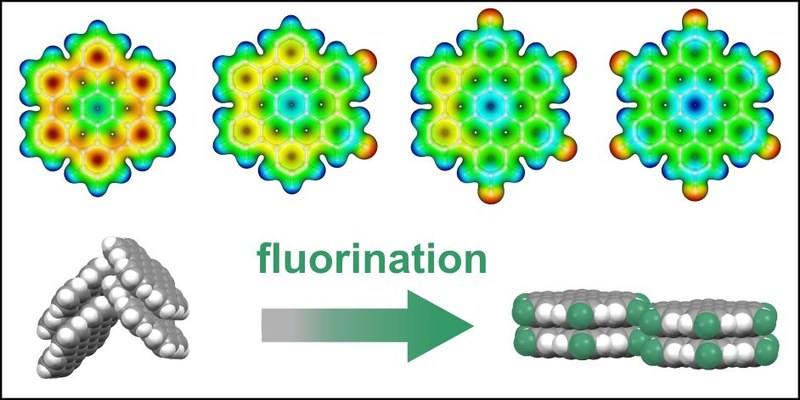
T. Breuer, M. Klues, P. Liesfeld, A. Viertel, M. Conrad, S. Hecht, G. Witte
Physical Chemistry Chemical Physics 18 (48), 33344-33350 (2016), DOI: 10.1039/C6CP06126E
We report on the synthesis and structural characterization of novel, partially fluorinated hexabenzocoronene (HBC) derivatives. Fluorination of polycyclic aromatic hydrocarbons (PAHs) is a well-established method to enhance the stability of organic semiconductors (OSCs) and render them n-type. For HBC it has been observed that fluorination leads to a modification of the molecular packing motif from a herringbone arrangement to a parallel-packed motif. Here, we study whether this transformation of the molecular packing is also found for the partially fluorinated HBCs 2,5-difluoro-hexaperi-hexabenzocoronene (F2HBC) and 2,5,8,11-tetrafluoro-peri-hexabenzocoronene (F4HBC). Combining powder diffraction and NEXAFS dichroism measurements, we reveal that indeed all partially fluorinated compounds adopt a parallel molecular packing, hence maximizing the intermolecular contact area. We identify fluorine-hydrogen bonds as mediating driving force to specifically stabilize this molecular arrangement and direct self-assembly. Furthermore, we show that the relative orientation of the HBCs on the underlying surface can be precisely controlled by varying substrate materials. Finally, the energetic states of the compounds are analyzed by photoelectron spectroscopy, optical spectroscopy and density functional theory to identify the effects of fluorination on these fundamental electronic characteristics.Inhalt ausklappen Inhalt einklappen CRYST. GROW. DES.: "Microstructural Analysis of Perfluoropentacene Films on Graphene and Graphite: Interface-Mediated Alignment and Island Formation"
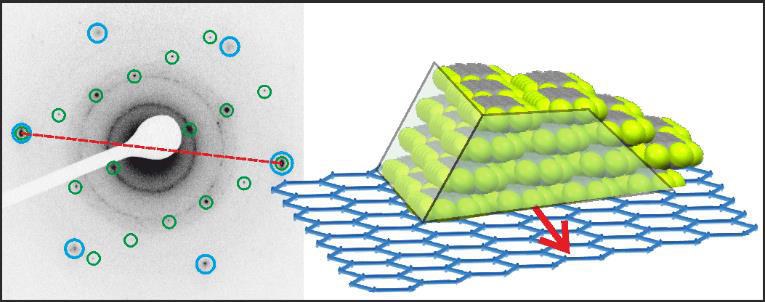
F. Rocio, T. Breuer, P. Rotter, F. Widdascheck, B. Eckhardt, G. Witte, K. volz, K. Gries
Crystal Growth & Design 16 (12), 6941-6950 (2016), DOI: 10.1021/acs.cgd.6b01117
The epitaxial growth of the n-type organic semiconductor perfluoropentacene (C22F14, PFP) on graphite and graphene substrates is analyzed by combining X-ray diffraction (XRD), scanning tunneling microscopy (STM), atomic force microscopy (AFM) and transmission electron microscopy (TEM). On both, graphite and graphene substrates, PFP forms islands where molecules adopt the π-stacked polymorph with lying molecular orientation relative to the substrate. By performing azimuthal analyses we identify the epitaxial relation between the PFP islands and the graphite substrate and gain insights into the crystal habitus of the PFP islands. Combining low-temperature STM measurements with molecular mechanics calculations, we provide a consistent mechanism for the azimuthal alignment at the PFP/graphene interface and the lateral positioning of the organic adsorbate on the graphene substrate.Inhalt ausklappen Inhalt einklappen PHYS. STAT. SOLIDI: "Structure of van der Waals bound Hybrids of Organic Semiconductors and Transition Metal Dichalcogenides: the Case of Acene Films on MoS2"
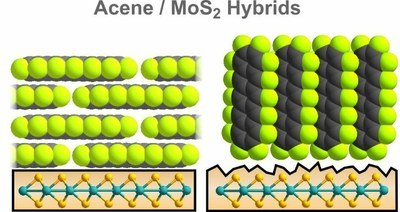
T. Breuer, T. Massmeyer, A. Mänz, S. Zoerb, B. Harbrecht, G. Witte
Physica Status Solidi - Rapid Research Letter 10 (12), 905-910 (2016), DOI: 10.1002/pssr.201600320
Transition metal dichalcogenides (TMDC) are important representatives in the emerging field of two-dimensional materials. At present their combination with molecular films is discussed as it enables the realization of van der Waals bound organic/inorganic hybrids which are of interest in future device architectures. Here, we discuss the potential use of molybdenum disulfide (MoS2) as supporting substrate for the growth of well-defined, crystalline organic adlayers. By this means, hybrid systems between the TMDC surface and organic compounds can be prepared, allowing for the profound investigation of mutual optical and electronic coupling mechanisms. As model system, we choose pentacene and perfluoropentacene as prototypical organic semiconductors and analyze their film formation on MoS2(001) surfaces. In both cases, we observe smooth, crystalline film growth in lying molecular configuration, hence enabling the preparation of well-defined hybrid systems. By contrast, on defective MoS2 surfaces both materials adopt an upright molecular orientation and exhibit distinctly different film morphologies. This emphasizes the importance of highly ordered TMDC surfaces with low defect density for the fabrication of well-defined hybrid systems.Inhalt ausklappen Inhalt einklappen Langmuir: "Patterned Growth of Organic Semiconductors: Selective Nucleation of Perylene on Self-Assembled Monolayers"
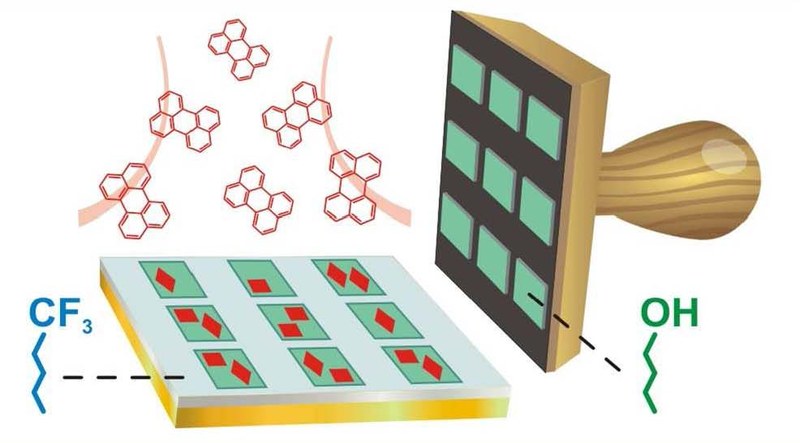
A. Pick, g. Witte
Langmuir 32 (32), 8019-8028 (2016), DOI: 10.1021/acs.langmuir.6b01833
Organic semiconductors (OSC) have attracted large interest because they afford the fabrication of flexible electronic devices. How-ever, the limited resistance to radiation and etching of such materials does not permit their patterning by photolithography, which has been a driving force for the development of integrated circuits, and therefore requires alternative structuring techniques. One approach is based on pre-coating the substrate with self-assembled monolayers (SAMs) to control the nucleation of subsequently depos-ited OSC layers, but the underlying mechanism is yet barely understood. Here, we used alkanethiols of different chemical termination to prepare SAMs on gold substrates serving as model systems to identify the mechanism of selective nucleation for the case of the OSC perylene. Using atomic force microscopy and fluorescence microscopy, we demonstrate that the chemical functionalization of the SAMs determines the adhesion forces to the OSC which are smallest for CF3-terminated and largest for OH-terminated SAMs, hence yielding distinctly different sticking-probabilities upon perylene deposition at room temperature. Micro-contact printing and immersion was employed to prepare SAM patterns that enable a selective growth of poly-crystalline perylene films. A quite different situation is found upon printing of long chain thiols with low vapor pressure which leads to the transfer of multilayers and favors the growth of perylene single crystallites. In a more abstract scenario patterns of silicone oil droplets were printed onto a gold substrate, which was afore covered with a repelling fluorinated SAM. Such droplets provide nucleation centers for liquid-mediated growth often yielding platelet-shaped perylene single crystallites without unwanted perylene nucleation on the remaining surface.Inhalt ausklappen Inhalt einklappen J. PHYS. CHEM. C: "Understanding the F1s NEXAFS Dichroism in Fluorinated Organic Semiconductors"
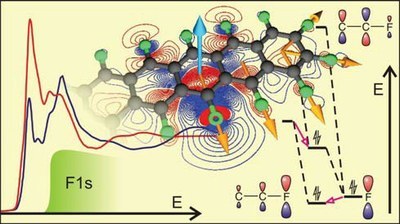
M. Klues, P. Jerabek, T. Breuer, M. Oehzelt, K. E. Hermann, R. Berger, G. Witte
Journal of Physical Chemistry C 120 (23), 12693-12705 (2016), DOI: 10.1021/acs.jpcc.6b04048
The analysis of NEXAFS spectra acquired at the absorption edge of heteroatoms like fluorine, nitrogen or oxygen enables the determination of the molecular orientation of individual compounds even in multinary structures. Such an analysis requires detailed knowledge about the nature of the corresponding electronic excitations, especially because 1s-π* and 1s-σ* excitations frequently feature disparate transition dipole moments in planar molecules. Unlike intuitively assumed, the resulting dichroisms of both excitation types in planar systems are, however, not necessarily inverted. Instead, both, the structure of the molecules and their alignment on the substrate determine the actual dichroisms. In this study, the NEXAFS signature and dichroisms of thin films of the organic n-type semiconductor perfluoropentacene (PFP) in different crystalline orientations at the carbon and fluorine absorption K-edge are thoroughly analyzed. For lying molecular orientations, an inverted dichroism at the fluorine K-edge compared to the carbon K-edge is found. In contrast, for upright molecular configurations the dichroisms at both absorption edges are similar. With the help of density functional theory methods, this behavior is explained by the different nature of the excitations. While at the carbon K-edge, 1s-π*-excitations are most prominent, 1s-σ*-related signals are dominant at the fluorine K-edge. Computations of the NEXAFS signatures and corresponding excitations at different levels of theory are compared with particular focus on electronic relaxation. Energetic positions and oscillator strengths, especially for resonances of σ*-type, depend strongly on the theoretical approach whereas the π*-related signals are barely affected. Similar effects were also found for the analysis of the smaller perfluoronaphthalene and are explained by the different relaxation effects for 1s-σ*- and 1s-π*-type excitations. As the investigated acenes are representative model systems for π-conjugated molecular semiconductors, the present findings are important for the understanding of the electronic properties and the application of NEXAFS for structural analysis in such materialsInhalt ausklappen Inhalt einklappen ACS APPL. MATER. INTERFACES: "Etching of Crystalline ZnO Surfaces upon Phosphonic-Acid Adsorption: Guidelines for the Realization of Well-Engineered Functional Self-Assembled Monolayers"
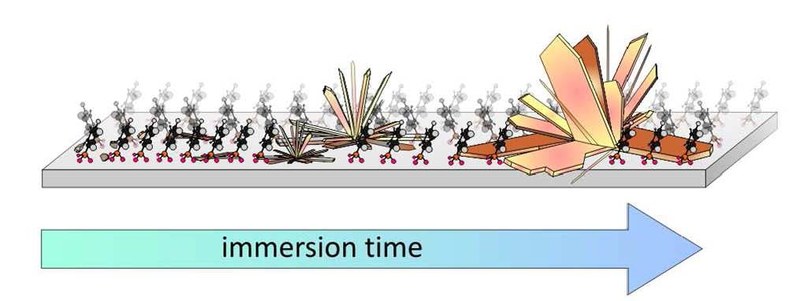
A. Ostapenko, T. Klöffel, J. P. Fußner, K. Harms, S. Dehnen, B. Meyer, G. Witte
ACS Applied Materials & Interfaces 8 (21), 13472-13483 (2016), DOI: 10.1021/acsami.6b02190
Functionalization of metal oxides by means of covalently bound self-assembled monolayers (SAMs) offers a tailoring of surface electronic properties, such as their work function, and in combination with its large charge carrier mobility renders ZnO a promising conductive oxide for use as transparent electrode material in optoelectronic devices. In this study we show that the formation of phosphonic acid anchored SAMs on ZnO competes with an unwanted chemical side reaction leading to the formation of surface precipitations and severe surface damage at prolonged immersion times of several days. Combining atomic force microscopy (AFM), X-ray diffraction (XRD) and thermal desorption spectroscopy (TDS), the stability and structure of the aggregates formed upon immersion of ZnO single crystal surfaces of different orientation [(0001), (0001) and (1010)] in phenylphosphonic acid (PPA) solution were studied. By intentionally increasing the immersion time to more than a week, large crystalline precipitations are formed which are identified as zinc phosphonate. Moreover, the energetics and the reaction pathway of this transformation has been evaluated using density functional theory (DFT) showing that zinc phosphonate is thermodynamically more favorable than phosphonic acid SAMs on ZnO. A formation of precipitations is also found for phosphonic acids with fluorinated aromatic backbones, while fewer precipitations are formed upon formation of SAMs with phenylphosphinic anchoring units. By contrast, no precipitations are formed when PPA monolayer films are prepared by sublimation under vacuum conditions, yielding smooth surfaces without noticeable etching.Inhalt ausklappen Inhalt einklappen LANGMUIR: "Formation and Stability of Phenylphosphonic Acid Monolayers on ZnO: Comparison of in-situ and ex-situ SAM Preparation"
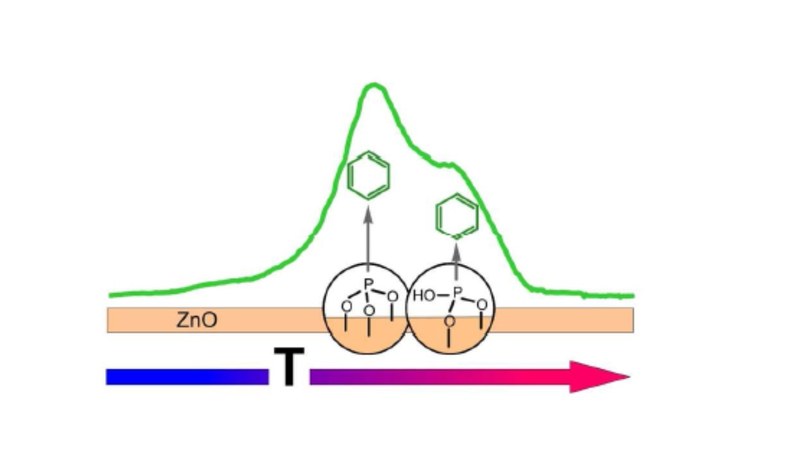
A. Ostapenko, T. Klöffel, B. Meyer, G. Witte
Langmuir 32 (20), 5029-5037 (2016), DOI: 10.1021/acs.langmuir.6b00487
Self-assembled monolayers (SAMs) enable an electronic interface tailoring of conductive metal oxides and offer an alternative to common transparent electrodes in optoelectronic devices. Here,the influence of surface orientation and pretreatment on the formation and stability of SAMs has been studied for the case of phenylphosphonic acid (PPA) on ZnO single crystals. Using thermal desorption spectroscopy (TDS), X-ray photoelectron spectroscopy (XPS), near-edge X-ray adsorption fine structure spectroscopy (NEXAFS) and density-functional theory (DFT) calculations, the thermal stability and orientational ordering of PPA-SAMs on the polar and mixed terminated ZnO surfaces were analyzed. On all surfaces PPA-SAMs remain stable up to 550 K, while at higher temperatures a C-P bond cleavage and dissociative desorption takes place yielding two distinct desorption peaks. Based on DFT calculations these desorption channels are attributed to protonated and deprotonated chemisorbed PPA molecules which can be related to tri- and bidentate species, hence allowing to determine their relative abundance from the intensity ratio. Beside immersion, an alternative monolayer preparation based on vacuum deposition in combination with controlled desorption of excess multilayers is demonstrated. This enables a SAM preparation on bare ZnO surfaces without any pre-coating due to exposure to ambient air, which is further compared with SAM formation on intentionally hydroxylated substrates. Corresponding TDS data indicate that initial hydroxylation favors the formation of tridentate and deprotonated bidentate, while the OMBD preparation on bare surfaces yields a larger fraction of protonated bidentate species. The orientation of PPA molecules adopted in the SAMs was determined from the dichroism of K-edge NEXAFS measurements and reveals an almost upright orientation for the deprotonated species, while a slight tilting is obtained for monolayer films with a large fraction of protonated bidentate molecules.Inhalt ausklappen Inhalt einklappen CRYSTENGCOMM.: "Exploration of MOF nanoparticles sizes using various physical characterization methods – Is what you measure what you get?"
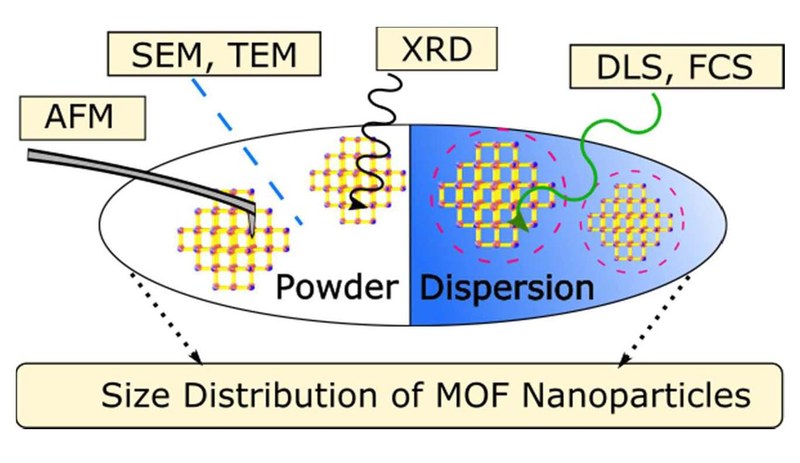
P. Hirschle, T. Preiß, F. Auras, A. Pick, J. Völkner, D. Valdeperez, G. Witte, W. Parak, J. O. Rädler, S. Wuttke
CrystEngComm. 18, 4359-4368 (2016), DOI: 10.1039/C6CE00198J
While the size of nanoparticles (NPs) seems to be a concept fixed in the field of NPs and is commonly used to characterize them, its definition is not that trivial as different “sizes” have to be distinguished depending on the physical characterization technique performed to measure them. Metal-organic frameworks (MOFs) are known for their crystallinity, their large variety of composition due to a huge number of inorganic building blocks that can be combined with almost endless organic linkers, their tunable pore structure, their ultrahigh porosity, and the different ways their backbones can be functionalized. The combination of these features with the nanoworld offers manifold perspectives for the synthesis of well-defined MOF nanoparticles (NPs), whose size attribute should be accurately determined as it strongly influences their physico-chemical properties (at this length scale). To elucidate the challenge of size determination for MOF NPs, zirconium fumarate MOF NPs (Zr-fum MOF NPs) were prepared and characterized using common physical characterization tools. Solid-state methods, including XRD, AFM, SEM and TEM were performed and their results were compared to the ones of dispersion-based methods as FCS and DLS. In doing so, we illustrate the difficulties of finding the appropriate method to determine the proper MOF NP size. We also demonstrate the importance of applying multiple complementary techniques as soon as MOF NP size is considered. Throughout this paper, we highlight and define some reasonable recommendations of how the MOF NP size should be explored.Inhalt ausklappen Inhalt einklappen NATURE MATER.: "Coupling between diffusion and orientation of pentacene molecules on an organic surface"
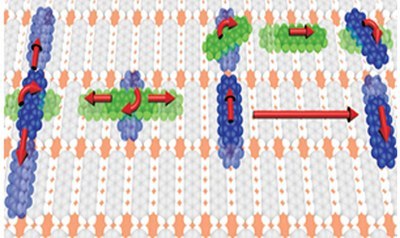
P. Rotter, B. A. J. Lechner, A. Morherr, D. M. Chismall, D. J. Ward, A. P. Jardine, J. Ellis, W. Allison, B. Eckhardt, G. Witte
Nature Materials 15 (4), 397-400 (2016), DOI: 10.1038/nmat4575
The realization of efficient organic electronic devices requires the controlled preparation of molecular thin films and heterostructures. As top-down structuring methods such as lithography cannot be applied to van der Waals bound materials, surface diffusion becomes a structure-determining factor that requires microscopic understanding. Scanning probe techniques provide atomic resolution, but are limited to observations of slow movements, and therefore constrained to low temperatures. In contrast, the helium-3 spin-echo (HeSE) technique achieves spatial and time resolution on the nm and ps scale, respectively, thus enabling measurements at elevated temperatures. Here we use HeSE to unveil the intricate motion of pentacene admolecules diffusing on a chemisorbed monolayer of pentacene on Cu(110) that serves as a stable, well-ordered organic model surface. We find that pentacene moves along rails parallel and perpendicular to the surface molecules. The experimental data are explained by admolecule rotation that enables a switching between diffusion directions, which extends our molecular level understanding of diffusion in complex organic systems.Inhalt ausklappen Inhalt einklappen J. PHYS. CHEM. C: "Enhanced Stability of Rubrene against Oxidation by Partial and Complete Fluorination"
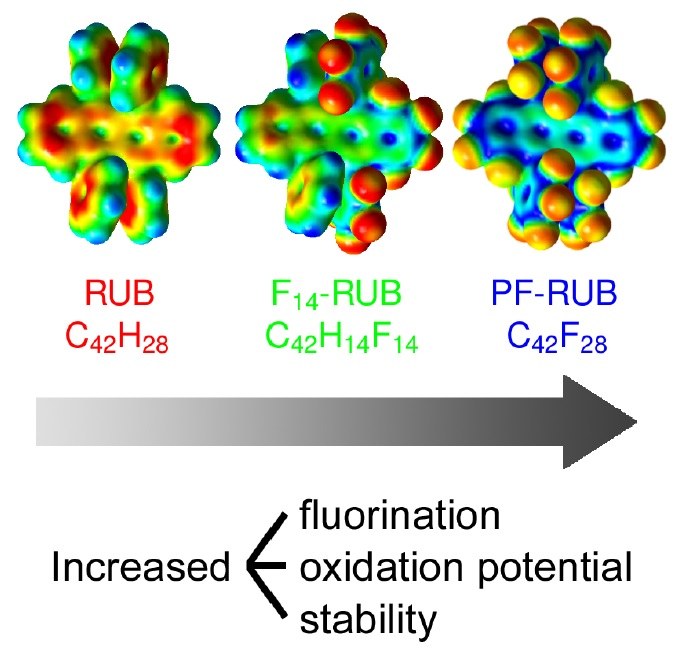
F. Anger, T. Breuer, A. Ruff, M. Klues, A. Gerlach, R. Scholz, S. Ludwigs, G. Witte, F. Schreiber
Journal of Physical Chemistry C 120 (10), 5515-5522 (2016), DOI: 10.1021/acs.jpcc.5b12293
We report on the oxidation potential of partially fluorinated (C42F14H14, F14-RUB) and perfluorinated rubrene (C42F28, PF-RUB) studied by cyclic voltammetry (CV) in solution as well as by spectroscopic ellipsometry and near edge X-ray absorption fine structure (NEXAFS) spectroscopy in thin films in combination with density functional theory computations. Due to their different electronic structure, the fluorinated derivatives have a higher oxidation potential and are more stable than rubrene (C42H28, RUB).Inhalt ausklappen Inhalt einklappen PHYS. CHEM. CHEM. PHYS.: "Temperature-Resolved Optical Spectroscopy of Pentacene Polymorphs: Variation of Herringbone Angles in Single-Crystals and Interface-Controlled Thin Films"
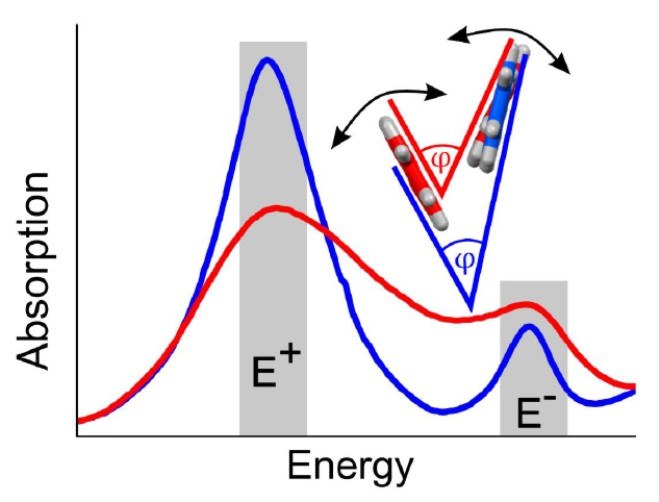
I. Meyenburg, T. Breuer, A. Karthäuser, S. Chatterjee, G. Witte, W. Heimbrodt
Physical Chemistry Chemical Physics 18 (5), 3825-3831 (2016), DOI: 10.1039/C5CP07836A
The polarization-resolved absorption spectra are determined for different pentacene polymorphs, both, for thin films grown on ZnO as well as for free-standing single crystals. A clear interrelation between the Davydov splitting of the lowest-energy singlet-exciton type transitions and the herringbone angle of the molecules in the unit cell is found. The variation in oscillator strength of the individual excitonic Davydov components with temperature is explained by a variation of this herringbone angle. The extraordinarily strong variation of the herringbone angle for Campbell phase pentacene films grown on ZnO substrates is attributed to interface-mediated strain due to the different thermal expansion coefficients of the organic and inorganic constituents.Inhalt ausklappen Inhalt einklappen ADV. MATER. INTERFACES: "Effects of Molecular Orientation in Acceptor-Donor Interfaces between Pentacene and C60 and Diels-Alder Adduct Formation at the Molecular Interface"
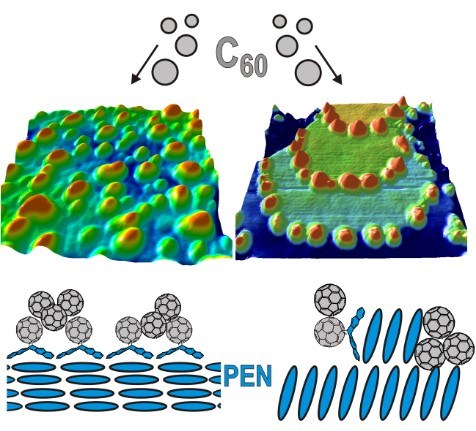
T. Breuer, A. Karthäuser, G. Witte
Advanced Material Interfaces 3 (7), 1500452 (2016), DOI: 10.1002/admi.201500452
Interfaces between pentacene and Buckminster-Fullerene (C60) have attracted interest due to their application as oligomeric model system for organic solar cells. As the actual device characteristics in such implementations are crucially controlled by the interface structure, detailed investigations of this interface on a molecular level are mandatory. In this study we analyze the influence of the orientation of the pentacene molecules in highly-ordered crystalline bottom layers on the characteristics of such internal interfaces. We show that the interface structure is driven by temperature-controlled diffusion of C60 molecules to the pentacene step-edges in the case of uprightly-oriented pentacene. For lying pentacene in the bottom layer, no step-edge decoration is observed while the wetting of the pentacene layer is enhanced. Furthermore, the stability of the interface against intercalation and re-orientation has been analyzed by means of NEXAFS spectroscopy, showing that the orientation of the pentacene molecules at the interface remains unchanged. Instead, we observe strong indication for chemical modification of the molecular entities by the formation of Diels-Alder adducts between C60 and pentacene. Finally, we show that C60 forms crystalline islands in thicker films only on top of uprightly-oriented pentacene while rather amorphous films are formed on lying pentacene.