Developmental Genetics
Shaping cells, tissues and embryos
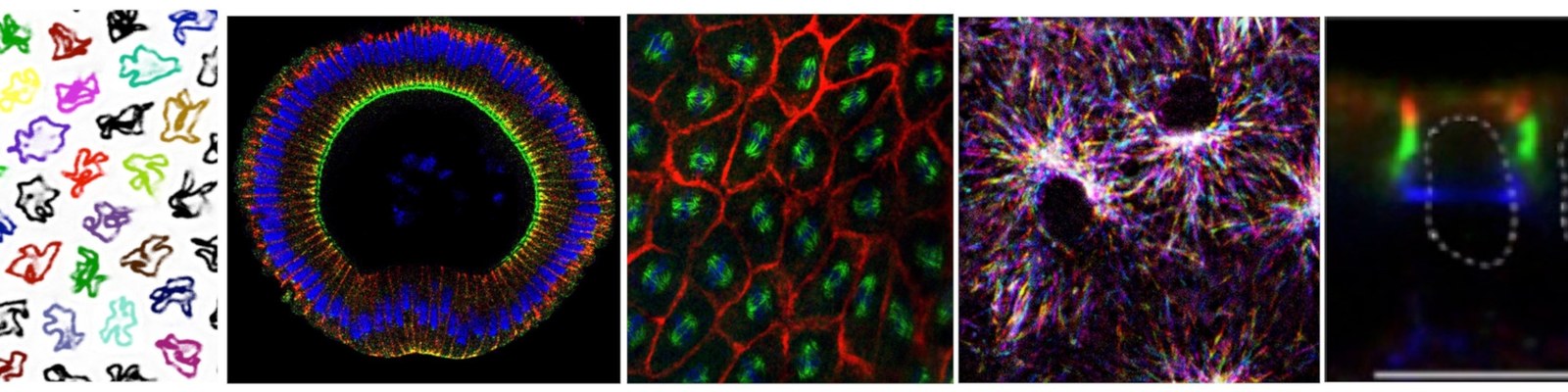
How do cells and tissue acquire their typical polarity, shape? During development cells proliferate, undergo cell shape changes and rearrange within the tissue to finally generate the stereotypic morphology of the embryo. The tight spatiotemporal control and coordination of these morphogenetic processes is essential for development, and loss of control or coordination of them is a hallmark in the pathology of many diseases.
Our research focuses on early Drosophila embryos, which provide a number of conceptual and experimental advantages, given the high speed of development, simple morphology as well genetic and microscopic tractability. We study the factors and molecular mechanisms controlling the transition from fast to slow cell cycles, forming and polarising epithelial tissue, orchestrating cell rearrangement and coordinating behaviour between neighbouring cells. We have discovered and analysed factors linking cell cycle control and morphogenesis, epithelial polarisation and cell coordination. Beside state-of-the-art molecular and transgenic approches, we are extensively using and developing procedures for high-resolution microscopy to track cells, cell structure and molecules in living embryos. We are employing and developing opto-genetic and opto-chemical methods as well as invasive UV laser based microsurgery to reveal and interfere with biophysical parameters of cells and tissues.
CONTACT:
Prof. Dr. Jörg Großhans, email: joerg.grosshans@biologie.uni-marburg.de, Phone +49-6421-282-21502, Mailing address: Philipps University, Grosshans lab, Department of Biology, Hans-Meerwein-Str. 6, 35043 Marburg, Germany