Main Content
Phosphoinositide Signaling
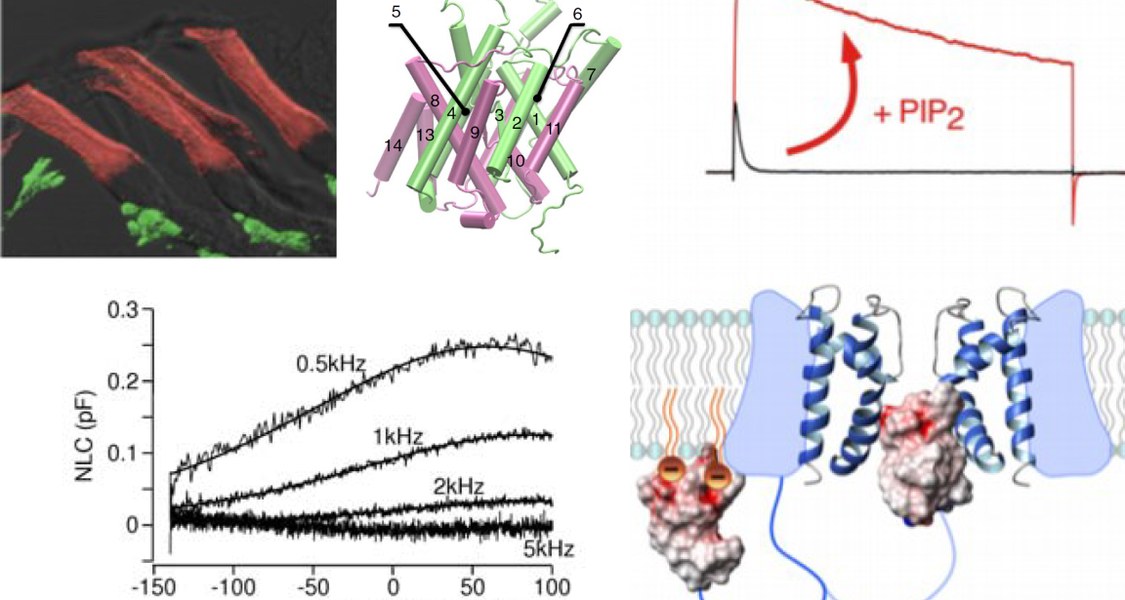
Many cellular processes, such as membrane fusion, actin dynamics, and the activity of membrane transporters and ion channels are controlled by certain lipid components of the membrane. We are interested in the phosphoinositides (PIs), a class of phospholipids, which are minor components of all cellular membranes and have important and diverse roles in the control of protein function and as organelle-specific membrane labels.
Recent work indicates that PIs are powerful modulators of many different ion channels. We have shown that PI(4,5)P2 can convert rapidly inactivating potassium channels (so-called A-type channels) into non-inactivating, delayed-rectifier-type channels (Oliver et al., 2004). Such change of channel behaviour is expected to dramatically alter the excitability of neurons.
It is also clear that through dynamic concentration changes resulting from breakdown or interconversion of PI isoforms, PIs act as bona-fide second messengers. PI dynamics can occur in response to a large variety of cellular events, including the activation of plasma membrane receptors.
Remarkably little, however, is still known about the dynamics of phosphoinositides in neurons (and most other cell types). Therefore, our main focus is to elucidate these presumptive PI dynamics under physiological conditions. For example, to what extent and where in the neuron does the plasma membrane PI(4,5)P2 level change when the neuron receives synaptic input acting on metabotropic neurotransmitter receptors?
Finally, we want to understand how in turn these PIs dynamics control the neuron’s electrical behaviour by modulating ion channel activity.
For the analysis of spatiotemporal PI dynamics, we use cellular imaging approaches that include total internal reflection fluorescence (TIRF) microscopy and confocal microscopy of living neurons. Changes in PI concentrations are detected by genetically encoded fluorescent probes that are based on PI-binding protein domains fused to spectral variants of GFP.