Main Content
Intercellular signaling mediated by pro-inflammatory IL-17A/F in ovarian cancer
Funding: DFG GRK2573/1, RP2
Ovarian cancer is the most lethal gynecological malignancy, which is accompanied in advanced stages by an accumulation of peritoneal effusion termed ascites. This microenvironment specific for ovarian carcinoma contains soluble mediators secreted by residual cells including tumor cells, activated mesothelial cells, adipocytes and immune cells. The peritoneal fluid contributes directly to the dissemination of tumor cells to the omentum and peritoneum. Therefore, understanding the cellular source, regulation of production and influence of mediators is important for understanding of disease underlying mechanisms.
The cytokines IL-17A/F act via the receptor composed of two subunits, the common chain for different IL-17 cytokine family members IL-17RA and IL-17RC, which is specific for IL-17A/F. The regulation of IL-17RC expression is not exactly understood, however, it has recently been reported that FSTL-1 induces IL-17RC expression in stromal cells. Furthermore, the soluble IL-17RC has been suggested to mediate “trans-signaling” to cells, which lack IL-17RC but express IL-17RA. The signaling via IL-17RA/C induces activation of several pathways including NFκB, MAPK, and GSK3ß. IL-17A/F are produced by different immune cell populations including CD4+ T (Th17) cells, CD8+ (Tc17) cells, γδT cells, some natural killer (NK) cells, and type 3 innate lymphoid cells (ILC3). The production of these cytokines is differentially regulated in each cell type. Thus, understanding which population is a source of IL-17A, is important for elucidating disease mechanisms. Besides the detrimental role of IL-17A/F in autoimmunity of the central nervous system and psoriasis, IL-17A promotes tumorigenesis in colorectal, pancreatic, mammary and ovarian carcinoma. However, the underlying mechanisms are poorly understood.
The cytokines IL-17A and IL-17F mediate their activities via the IL-17 receptor (IL-17R) complex, consisting of the IL-17RA and IL-17RC subunits. IL-17RC triggers multiple pro-inflammatory signal transduction pathways, notably NFκB. Our preliminary data show that high expression of soluble IL-17RC in ascites is strongly associated with poor relapse free survival (RFS) in ovarian carcinoma.
The expression of IL-17RC is positively regulated by follistatin-like protein 1 (FSTL-1), which according to our data is associated with an unfavorable RFS and strongly correlates with IL-17RC expression. Therefore, we aim to dissect the FSTL-1 - IL-17RC - IL-17A/F regulatory circuit and elucidate its role in ovarian carcinoma progression, as summarized in the following:
(i) Considering that the cytokines IL-17A/F can be produced by different immune cell types including CD4+, CD8+, γδT cells, NK cells and ILC3, we plan to identify the cellular source of IL-17A/F that impact ovarian cancer RFS.
(ii) We will analyze the regulation of IL-17RC expression by FSTL-1 on tumor cells as well as tumor-associated mesothelial cells, adipocytes and macrophages.
(iii) We will evaluate direct effects of IL-17A/F on tumor cells or indirect via secreted factors by tumor-associated host cells. To evaluate indirect effects, we will co-culture tumor cells with mesothelial cells, adipocytes and macrophages or expose tumor cells to the respective conditioned media. The biological effect on the analyzed tumor cells will include proliferation, invasion, EMT and stemness.
(iv) Finally, we will identify mediators in the IL-17A/F-induced secretomes in tumor and host cells that are associated with a short RFS, and will study the mechanisms of their induction to identify potential therapeutic targets and inhibitors able to dampen the production of the pro-tumorigenic mediators.
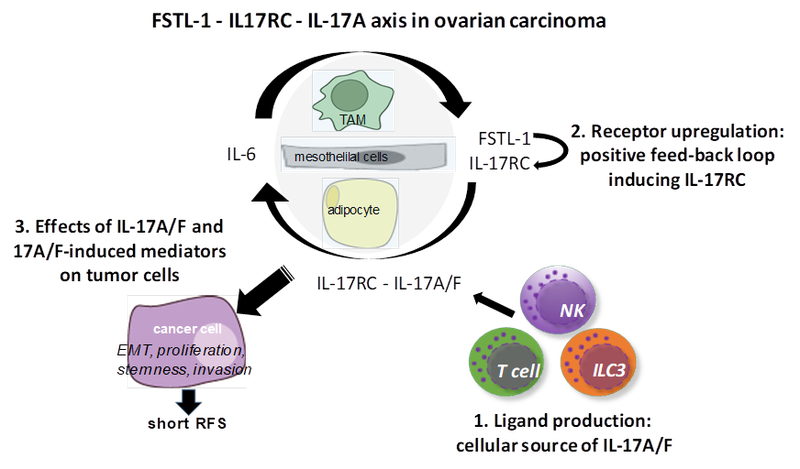
Figure 1. Work plan for dissecting FSTL-1-IL-17RC-IL-17A axis including three major steps (1-3) based on the preliminary data on the correlation of IL-17RC and FSTL-1 with short RFS in ovarian carcinoma. We follow the hypothesis that IL-6 upregulates FSTL-1, which induces IL-17RC expression, and in turn through a positive feedback loop IL-17RC-IL-17A/F signaling enhances IL-6 production by accessory cells. IL-17A/F via IL-17RC induces directly or indirectly enhanced tumorigenicity of cancer cells leading to shortened RFS. The source of IL-17A/F has to be defined.