Main Content
Subproject 8
Clinical phase Ib trial: T cell-targeted immunotherapy of pemphigus vulgaris
Summary
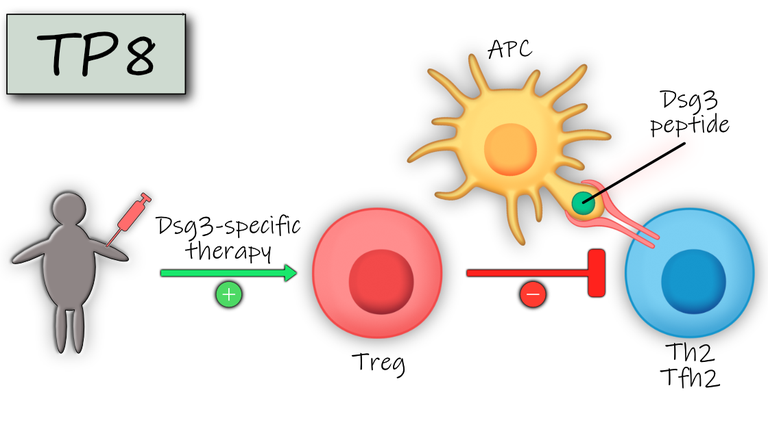
Pemphigus vulgaris (PV) is a severe prototypic autoantibody mediated skin disorder whose pathogenesis largely depends on the B cell help of autoreactive T helper (Th) cells that recognize epitopes of the PV autoantigen, desmoglein 3 (Dsg3), in association with the PV-associated HLA class II allele, HLA-DRB1*04:02. Currently, PV is treated with systemic immunosuppressants, such as glucocorticoids and adjuvants which leads to considerable co-morbidities on long-term treatment. Here we aim to therapeutically target autoreactive Th cells in PV with Dsg3 peptide-coupled tolerizing particles to induce a specific immune tolerance against Dsg3. Dsg3 T cell epitopes are attached to such particles which deliver their cargo to liver sinusoidal endothelial cells (LSEC). Embedded in the tolerogenic environment of the liver LSECs have the potential to convert naive and effector T cells into regulatory T cells. As shown recently, this immunotherapy led to the down regulation of the autoreactive, pathogenic T cell response in EAE, an animal model of multiple sclerosis, resulting in prevention of disease. In the anticipated phase Ia/b trial in PV we will not only study safety and toxicity of Dsg3 peptide immunotherapy but also thoroughly investigate the impact of this treatment on Dsg3-reactive pathogenic T cells and potentially regulatory T cells as well as other pro-inflammatory immune cells and humoral factors such as Dsg3-specific autoantibodies. The findings of this trial may also be applicable to other antibody-mediated human disorders which are regulated by autoreactive T cells.
Contact:

Philipps University Marburg
Prof. Dr. Michael Hertl
Project leader TP8, Speaker Pegasus
Clinic for Dermatology and Allergology
Baldingerstr.
D-35043 Marburg
Tel +49 06421 58 66281
Fax +49 06421 58 62902
Email: michael.hertl@med.uni-marburg.de
Inhalt ausklappen Inhalt einklappen Team
 Pegasus
PegasusDr. Dario Didona, Julia Hinterseher, Tomás Cunha, Dr. Karolin Wieber, Christine Zimmer PhD, Prof. Dr. Michael Hertl (from left to right)
Inhalt ausklappen Inhalt einklappen Publications of Project 8
Wieber K, Zimmer CL, Hertl M. Detection of autoreactive CD4+ T cells by MHC class II multimers in HLA-linked human autoimmune diseases. J Clin Invest. 2021 May 3;131(9):e148674. doi: 10.1172/JCI148674. PMID: 33938450; PMCID: PMC8087195.
Didona D, DI Zenzo G, Joly P. Paraneoplastic autoimmune multiorgan syndrome. Ital J Dermatol Venerol. 2021 Apr;156(2):174-183. doi: 10.23736/S0392-0488.20.06675-4. Epub 2020 Oct 16. PMID: 33070576.
Scarsella L, Pollmann R, Amber KT. Autoreactive T cells in pemphigus: perpetrator and target. Ital J Dermatol Venerol. 2021 Apr;156(2):124-133. doi: 10.23736/S0392-0488.20.06706-1. Epub 2020 Nov 12. PMID: 33179878.
Eming R, Zimmer CL, Hertl M. Pemphigus: a critical analysis on clinical subtypes, pathogenesis, diagnostics and established novel therapeutics. Ital J Dermatol Venerol. 2021 Apr;156(2):121-123. doi: 10.23736/S0392-0488.20.06790-5. Epub 2020 Dec 14. PMID: 33314892.
Didona D, Scarsella L, Fehresti M, Solimani F, Juratli HA, Göbel M, Mühlenbein S, Holiangu L, Pieper J, Korff V, Schmidt T, Sitaru C, Eming R, Hertl M, Pollmann R. Autoreactive Peripheral Blood T Helper Cell Responses in Bullous Pemphigoid and Elderly Patients With Pruritic Disorders. Front Immunol. 2021 Mar 25;12:569287. doi: 10.3389/fimmu.2021.569287. PMID: 33841390; PMCID: PMC8027500.
Didona D, Fania L, Di Zenzo G, Didona B. Erythromycin-induced pemphigus foliaceus successfully treated with etanercept. Dermatol Ther. 2020 Nov;33(6):e14201. doi: 10.1111/dth.14201. Epub 2020 Sep 11. PMID: 32808715.
Didona D, Fania L, Didona B, Eming R, Hertl M, Di Zenzo G. Paraneoplastic Dermatoses: A Brief General Review and an Extensive Analysis of Paraneoplastic Pemphigus and Paraneoplastic Dermatomyositis. Int J Mol Sci. 2020 Mar 21;21(6):2178. doi: 10.3390/ijms21062178. PMID: 32245283; PMCID: PMC7139382.
Didona D, Maglie R, Eming R, Hertl M. Pemphigus: Current and Future Therapeutic Strategies. Front Immunol. 2019 Jun 25;10:1418. doi: 10.3389/fimmu.2019.01418. PMID: 31293582; PMCID: PMC6603181.
Pollmann R, Walter E, Schmidt T, Waschke J, Hertl M, Möbs C, Eming R. (2019) Identification of autoreactive B cell subpopulations in peripheral blood of autoimmune patients with pemphigus. Front Immunol. 10:1375. doi: 10.3389/fimmu.2019.01375
Solimani F, Pollmann R, Schmidt T, Schmidt A, Savai R, Zheng X, Mühlenbein S, Pickert J, Eubel V, Möbs C, Eming R, Hertl M. Therapeutic targeting of Th17/Tc17 cells leads to clinical improvement of lichen planus. Front Immunol 2019. 10; doi:10.3389/ fimmu.2019.01808.
Kugelmann D, Rötzer V, Walter E, Egu DT, Fuchs MT, Vielmuth F, Vargas-Robles H, Schnoor M, Hertl M, Eming R, Rottner K, Schmidt A, Spindler V, Waschke J. Role of Src and Cortactin in Pemphigus Skin Blistering. Front Immunol. 2019 Apr 4;10:626. doi: 10.3389/fimmu.2019.00626. eCollection 2019.
Solimani F, Maglie R, Pollmann R, Schmidt T, Schmidt A, Ishii N, Tackenberg B, Pickert J, Kirschbaum A, Hashimoto T, Hertl M. (2019) Thymoma-associated paraneoplastic autoimmune multiorgan syndrome – from pemphigus to lichenoid dermatitis. Front Immunol. Case Report doi.org/10.3389/fimmu.2019. 01413
Didona D, Maglie R, Eming R, Hertl M. Pemphigus: current and future therapeutic strategies. Front Immunol. doi: 10.3389/fimmu.2019.01418 *shared authorship.
Amber KT, Maglie R, Solimani F, Eming R, Hertl M. Targeted Therapies for Autoimmune Bullous Diseases: Current Status. Drugs. 2018 Oct;78(15):1527-1548. doi: 10.1007/s40265-018-0976-5.
Schmidt T, Solimani F, Pollmann R, Stein R, Schmidt A, Stulberg I, Kühn K, Eming R, Eubel V, Kind P, Arweiler N, Sitaru C, Hertl M. TH1/TH17 cell recognition of desmoglein 3 and bullous pemphigoid antigen 180 in patients with lichen planus. J Allergy Clin Immunol. 2018 Aug;142(2):669-672.e7. doi: 10.1016/j.jaci.2018.02.044.
Pollmann R, Schmidt T, Eming R, Hertl M. Pemphigus: A Comprehensive Review on Pathogenesis, Clinical Presentation and Novel Therapeutic Approaches. Clin Rev Allergy Immunol. 2018 Feb;54(1):1-25. doi: 10.1007/s12016-017-8662-z.
Spindler V, Eming R, Schmidt E, Amagai M, Grando S, Jonkman MF, Kowalczyk AP, Müller EJ, Payne AS, Pincelli C, Sinha AA, Sprecher E, Zillikens D*, Hertl M*, Waschke J*. Mechanisms Causing Loss of Keratinocyte Cohesion in Pemphigus. J Invest Dermatol. 2018 Jan;138(1):32-37. doi: 10.1016/j.jid.2017.06.022.
Walter E, Vielmuth F, Rotkopf L, Sárdy M, Horváth ON, Goebeler M, Schmidt E, Eming R, Hertl M, Spindler V, Waschke J. Different signaling patterns contribute to loss of keratinocyte cohesion dependent on autoantibody profile in pemphigus. Sci Rep. 2017 Jun 15;7(1):3579. doi: 10.1038/s41598-017-03697-7.





