02.04.2025 Paper: Transport and Reaction of Electrons and Molecules in Solid Electrolyte Interphases formed at Different Electrode Potentials: A Combined Experimental and Modeling Approach
Falk Thorsten Krauss, Annalena Duncker, Prof. Dr. Bernhard Roling
Department of Chemistry, Philipps-Universität Marburg, Hans-Meerwein-Straße 4, 35032 Marburg, Germany
Abstract
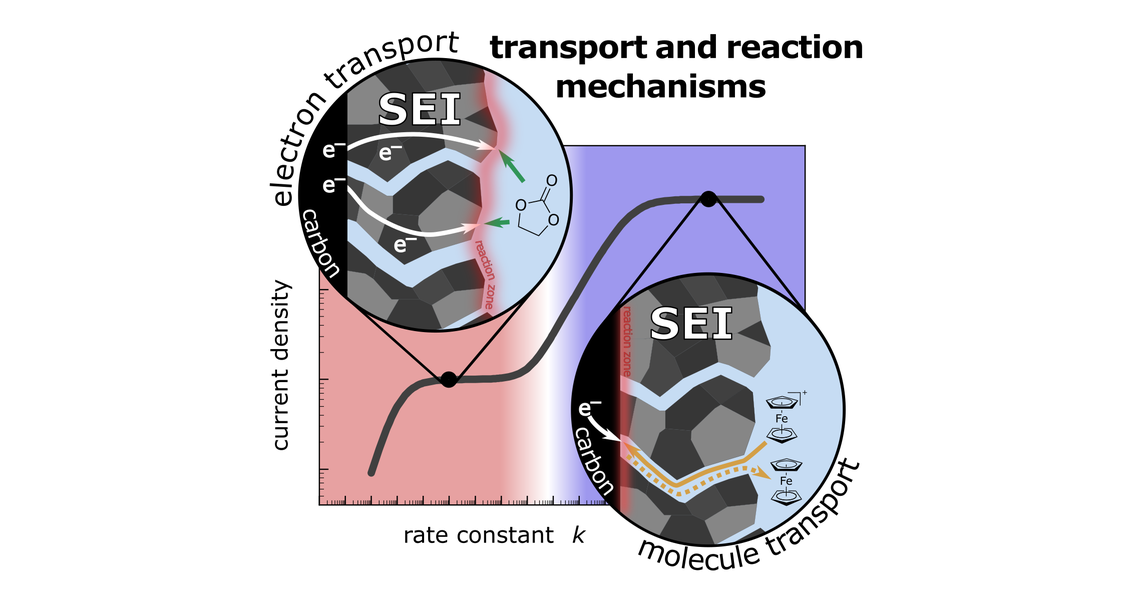
Good passivation properties of the solid electrolyte interphase (SEI) on the graphite-based negative electrode are essential for a long cycle life of lithium-ion batteries. Nevertheless, the underlying electron and molecule transport mechanisms inside the SEI are poorly understood. Here, we elucidate transport and reaction in model-type SEIs formed at different electrode potentials by combining generator-collector experiments and electrochemical impedance spectroscopy with a diffusion-reaction modeling approach. In the generator-collector experiments, we use a four-electrode-based setup to compare the electrolyte reduction current density with a redox molecule (ferrocenium Fc+) reduction current density at an SEI-covered glassy carbon electrode. We find that the current density ratio depends on the SEI formation potential as well as on the formation time. The experimental results are compared to the prediction of a transport and reaction model, which accounts for reduction reactions inside the SEI as well as in the double layer at the SEI | bulk electrolyte interface. This model predicts four distinct diffusion and reaction regimes depending on the rate constant for the molecule-electron reaction. Using this combined approach, we obtain good estimates for the transport coefficients of electrons and molecules inside the SEI.
Read the full paper in ChemSusChem 2025, 18, e202402468, published by Chemistry Europe.
The paper was celebrated with Cheesecake.