Main Content
Ion and Molecule Transport across the Solid Electrolyte Interphase (SEI)
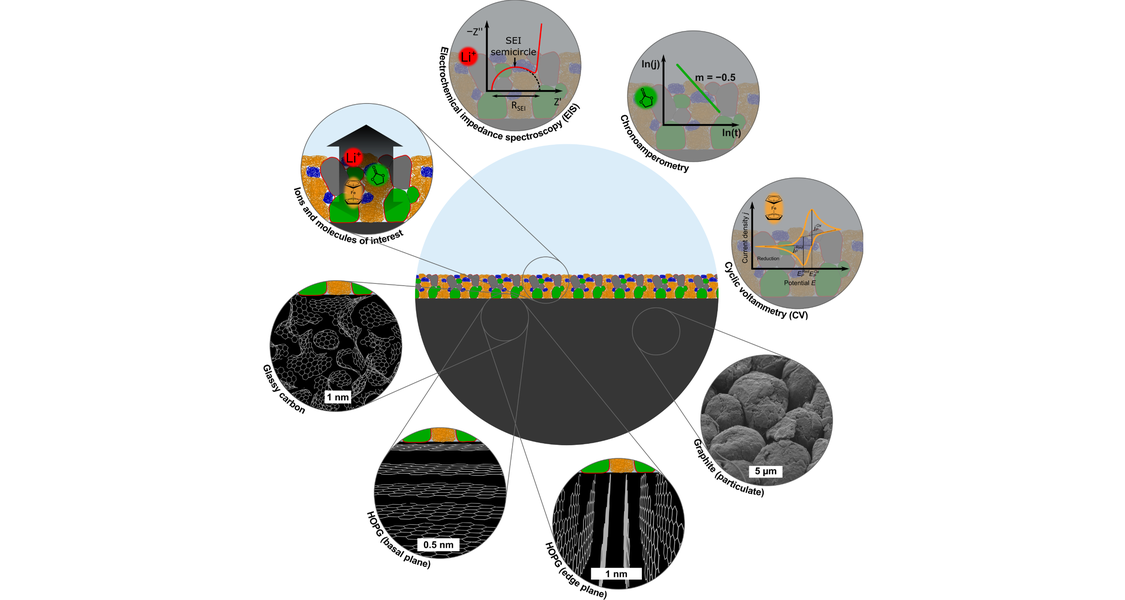
Summary:
Lithium ion batteries (LIBs) are widely used for mobile electronic devices. During the first charging cycle of the battery, the electrolyte (lithium salt dissolved in a mixture of organic carbonates) decomposes at the graphite anode / electrolyte interface. The decomposition products form the solid electrolyte interphase (SEI), which acts as a passivation layer preventing further electrolyte decomposition. In the ideal case, the SEI is a fast Li+ ion conductor and blocks electrons and molecules completely. However, real SEIs allow for a slow transport of electrons and/or molecules, which leads to further electrolyte decomposition and slow growing of the SEI. Due to the complex morphology of the SEI, the transport properties are not well understood up to now.
In order to elucidate the passivation properties of SEIs, we form model-type SEIs on planar model electrodes by electrochemical means and characterize their chemical composition, morphology and transport properties by combining a number of different experimental techniques (atomic force microscopy (AFM), focused ion beam scanning electron microscopy (FIB-SEM), cyclic voltammetry, electrochemical impedance spectroscopy and redox-probe experiments).
[1] P. J. F. Harris, Philos. Mag. 2004, 84 (29), 3159–3167.
Contact Persons:
Highlighted Publications:
- F. T. Krauss, I. Pantenburg, V. Lehmann, M. Stich, J. O. Weiershäuser, A. Bund, B. Roling, 'Elucidating the Transport of Electrons and Molecules in a Solid Electrolyte Interphase Close to Battery Operation Potentials Using a Four-Electrode-Based Generator-Collector Setup', Journal of the American Chemical Society 146 (2024), 19009. doi:10.1021/jacs.4c03029
- F. T. Krauss, I. Pantenburg, B. Roling, 'Transport of Ions, Molecules, and Electrons across the Solid Electrolyte Interphase: What Is Our Current Level of Understanding?', Adv. Mater. Interfaces (2022), 2101891. doi: 10.1002/admi.202101891
- S. Kranz, T. Kranz, A. G. Jaegermann, B. Roling, 'Is the solid electrolyte interphase in lithium-ion batteries really a solid electrolyte? Transport experiments on lithium bis(oxalato)borate-based model interphases', J. Power Sources 418 (2019) 138–146. doi: 10.1016.j.jpowsour.2019.01.060
- T. Kranz, S. Kranz, V. Miß, J. Schepp, B. Roling, 'Interrelation between Redox Molecule Transport and Li+ Ion Transport across a Model Solid Electrolyte Interphase Grown on a Glassy Carbon Electrode', J. Electrochem. Soc. 164 (2017) A3777–A3784. doi: 10.1149/2.1171714jes