Main Content
Ion Correlations in Concentrated Liquid Battery Electrolyte
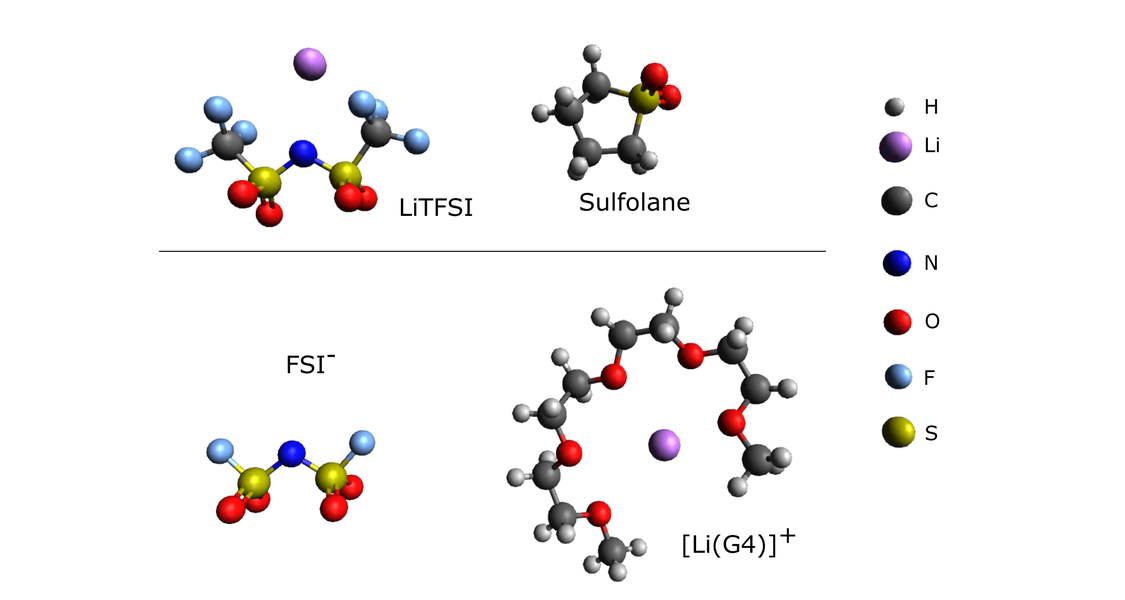
Summary:
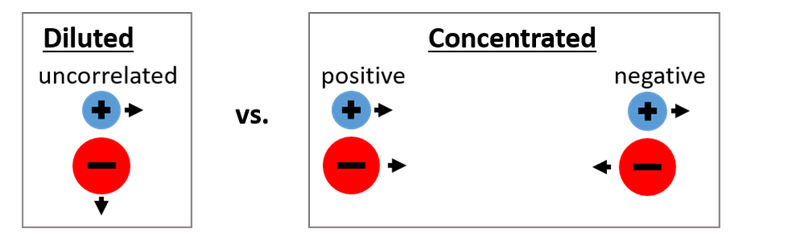
For the development of safer lithium ion batteries (LIBs), alternative liquid electrolytes with high salt concentrations are of great interest. In these concentrated electrolytes, most solvent molecules are bound in the solvation spheres of the ions, and only few “free” solvent molecules exist. This leads to a low vapor pressure and low flammability of these electrolytes. The high ion concentrations and the low number of “free” solvent molecules result in strong ion-ion interactions and in strong directional correlations between the ionic movements, see Fig. 2.
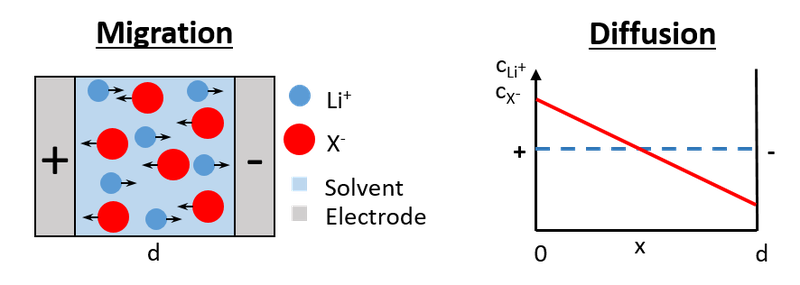
During the stationary charging and discharging of batteries, the Li+ ions move under anion-blocking conditions. In this case, a salt concentration gradient in the electrolyte builds up, such that the migration current and the diffusion current of the anions cancel exactly. It can be shown that the Li+ ion transport under these conditions is strongly influenced by the ion correlations sketched above. Both positive and negative correlations reduce the stationary Li+ current.
By measuring different electrolyte transport parameters, such as ionic conductivity (σion), Li+-transference number under anion-blocking conditions (tLi+) and salt diffusion coefficient (Dsalt), we obtain information about the ion correlations in concentrated electrolytes. To this end, we use different experimental methods, such as electrochemical impedance spectroscopy and concentration cell measurements.
Contact Persons:
Hightlighted Publications:
- H. Kilian, T. Pothmann, M. Lorenz, M. Middendorf, S. Seus, M. Schönhoff, B. Roling, 'Quantification of vehicular versus uncorrelated Li+–solvent transport in highly concentrated electrolytes via solvent-related Onsager coefficients', Phys. Chem. Chem. Phys. 27 (2025), 1593. doi: 10.1039/d4cp04209c
- T. Pothmann, M. Middendorf, C. Gerken, P. Nürnberg, M. Schönhoff, B. Roling, 'Overdetermination method for accurate dynamic ion correlations in highly concentrated electrolytes', Faraday discussions 253 (2024), 100. doi:10.1039/d4fd00034j
- S. Pfeifer, F. Ackermann, F. Sälzer, M. Schönhoff, B. Roling, 'Quantification of cation–cation, anion–anion and cation–anion correlations in Li salt/glyme mixtures by combining very-low-frequency impedance spectroscopy with diffusion and electrophoretic NMR', Phys. Chem. Chem. Phys. 23 (2021), 628–640. doi: 10.1039/D0CP06147F
- N. M. Vargas-Barbosa, B. Roling, 'Dynamic Ion Correlations in Solid and Liquid Electrolytes: How Do They Affect Charge and Mass Transport?', ChemElectroChem 7 (2020), 367–385. doi: 10.1002/celc.201901627
- D. Dong, F. Sälzer, B. Roling, D. Bedrov 'How efficient is Li+ ion transport in solvate ionic liquids under anion-blocking conditions in a battery?', Phys. Chem. Chem. Phys. 20 (2018) 29174–29183. doi: 10.1039/c8cp06214e
- F. Wohde, M. Balabajew, B. Roling, 'Li+ Transference Numbers in Liquid Electrolytes obtained by Very-low-frequency Impedance Spectroscopy at variable Electrode Distances', J. Electrochem. Soc. 163, 5 (2016) A714–A721. doi: 10.1149/2.0811605jes