Main Content
Crystalline and Amorphous Solid Electrolytes
Summary:
Solid electrolytes (SE) are advantageous over liquid electrolytes in terms of safety (incombustible and no battery bleeding) and versatility because they could eventually render Li+ or Na+ batteries operable at elevated temperatures compared to the respective liquid cells. One of the disadvantages of most solid electrolytes, however, is their oftentimes rather low conductivity, so that a high ionic conductivity becomes the most important prerequisite for a SE. There are a lot of different groups of SEs which are investigated nowadays: oxide-based electrolytes, for example LISICON and NASICON, polymer-based ones like for instance Li+TFSI– in poly ethylene oxide (PEO) or sulfide-based ones like Li3PS4. In our working group we are predominantly interested in the latter, more specifically in both crystalline – for instance Li4SnS4 and Li6PS5Cl – and amorphous (e.g. Li7P2S8I) sulfide-based SEs. In particular, we characterize the solid electrolyte and then try to examine its practicality for the use in all solid-state batteries (Characterization of All-Solid-State Batteries).
Characterization of solid electrolytes
Apart from the need for high ionic conductivities a competitive price is also an important requirement. To this end, our group came up with the idea to substitute Ge by Sn in the famous solid electrolyte Li10GeP2S12 (LGPS). In fact Li10SnP2S12 (LSnPS) assumes the same crystal structure with only minor differences in the occupation of the different Li+ positions. The resulting conductivity of LSnPS (7 mS/cm) is only slightly lower than for LGPS (14 mS/cm) but the replacement of Ge by Sn should reduce the raw material cost by a factor of roughly 3. This led to an even higher interest in this material class and a lot of research is still focused on LSnPS proving it to be still highly topical.
In addition to our research efforts in the field of Li+ electrolytes we are – in cooperation with the research group of Prof. Dr. Stefanie Dehnen – also investigating chalcogenide-based Na+ electrolytes. Even though a lot of the knowledge about Li+ conductors can be transferred to its “bigger chemical brother” not everything applies analogously. Recently, we have found an interesting new structural class of Na+ conductors with NaSnPS (Na11Sn2PS12) being its first member. Its stoichiometry deviates clearly from the similar but structurally still very different group of 1−4−5−6 Li+ electrolytes with its very prominent example: LGPS: Li10GeP2S12.
The conductivity of NaSnPS surpasses the ones observed for previously reported sulfide-based Na+ electrolytes but is not as high as for LGPS. Elemental substitutions that led to meanwhile reported structural relatives like NaSnSbS and NaSnPSe have not yet shown improved conductivities. But the field of Na+ electrolytes is still rather young and further substitutions are likely to foster the ionic conductivity in future works as it has been shown for the lithium counterparts.
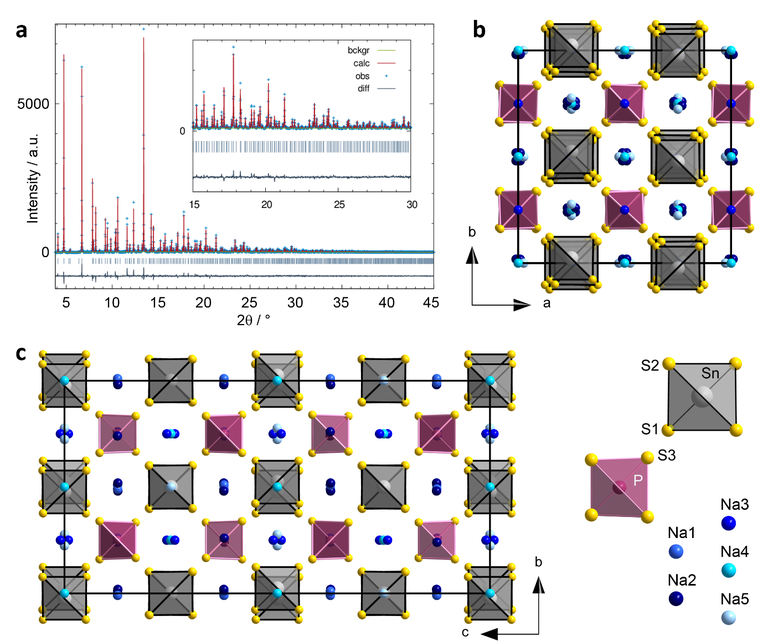
Figure 1: Crystal structure of Na11Sn2PS12. a) Rietveld refinement of the diffraction pattern. In the inset, an enlarged view of the high angle region is shown. b) Crystal structure viewed along [001]. c) Crystal structure viewed along [100]. Sn atoms as well as [SnS4]4– tetrahedra are drawn in grey, P atoms and [PS4]3– tetrahedra in purple, S atoms in yellow and Na+ cations in different shades of blue in order to differentiate between the five crystallographically distinct sites, as indicated in the legend beside
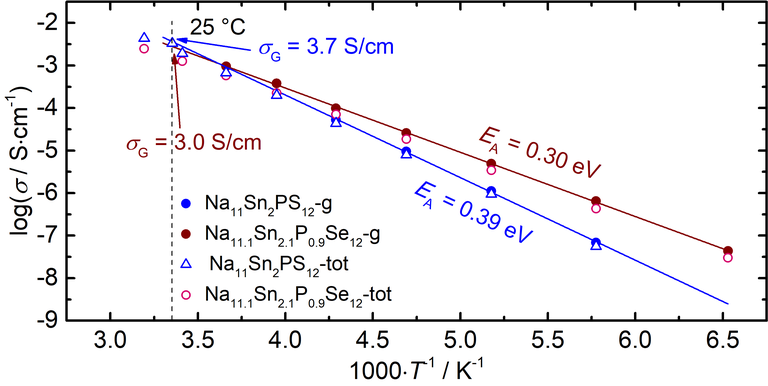
Figure 2: Arrhenius plot of the grain and total conductivities of NaSnPS and NaSnPSe with respective activation energies and grain conductivities at 25 °C.
Highlighted Publications:
- M. Duchardt, U. Ruschewitz, S. Adams, S. Dehnen, B. Roling, 'Vacancy-controlled Na+ Superion Conduction in Na11Sn2PS12', Angew. Chem. Int. Ed. 57 (2018) 1351-1355. doi: 10.1002/anie.201712769.
- P. Bron, S. Johansson, K. Zick, J. Schmedt auf der Günne, S. Dehnen, B. Roling, 'Li10SnP2S12: An affordable lithium superionic conductor', J. Am. Chem. Soc. 135 (2013), 15694-15697. doi: 10.1021/ja407393