Main Content
Acute depletion of Ush & Lint-1
Deborah Trummel
The chromatin structure is regulated by enzymatic as well as non-enzymatic mechanisms which play an essential role in the proliferation, differentiation and transformation of cells. Components of ATP-dependent chromatin remodelers such as dMi-2 and histone modifying protein complexes (e.g. the LINT complex) drive and shape these complex processes. DNA-binding proteins like Ush, a major transcriptome regulator, repress or activate the transcription of genes hereby enabling the development of cellular identity.
The presence and absence as well as the amount and the interaction with other epigenetic elements are key for the maintenance of healthy cells. Often the factors mentioned above are misexpressed or mutated in cancer and other diseases.
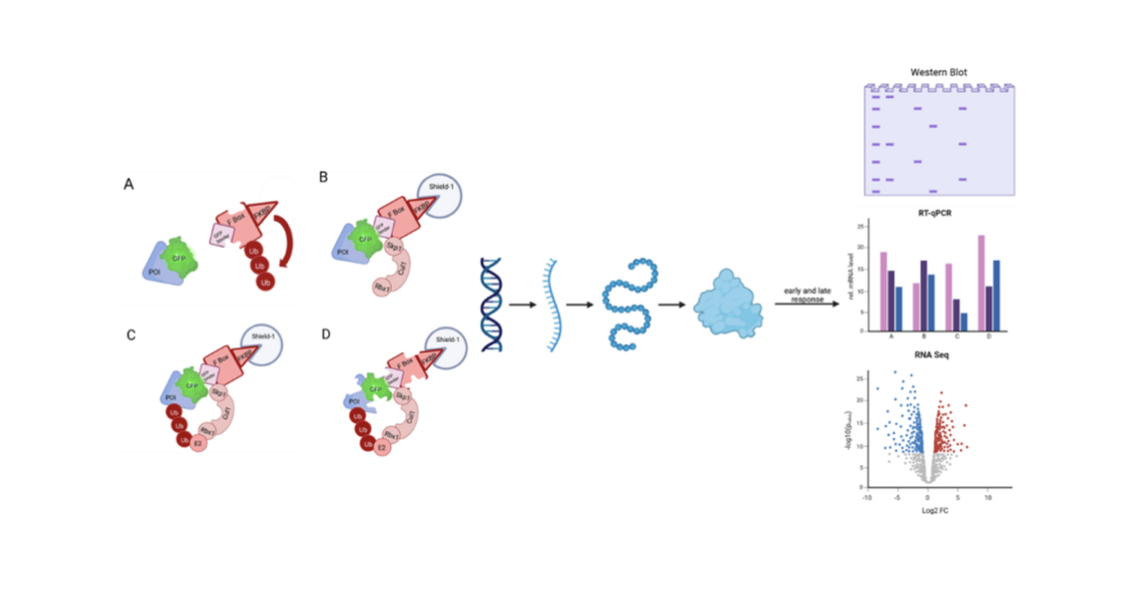
Regarding e.g. transcriptional processes time is an essential factor and knocking down POIs with RNAi constructs takes up to 4 days. It is not entirely clear whether the observed effects are caused by the protein loss or not. For example it is possible that these effects are compensated by other proteins. Therefore it is difficult to distinguish between direct and indirect impacts. An alternative approach to circumvent many of the problems described above is to induce the rapid degradation of a given POI.
Several methods have recently been developed for targeted protein degradation. In this PhD project one such method, deGradFP, has been established and used for rapid degradation of Ush and dLint-1. DeGradFP-mediated protein degradation has been shown to only require hours for certain proteins (Banaszynski et al., 2006; Caussinus et al., 2011; An et al., 2015). An induced rapid degradation of Ush and dLint-1 will allow me to address questions such as how fast does the gene expression program change after the depletion of these proteins? Is there an early and/or a late response? What are the underlying molecular mechanisms that affect gene expression changes? I am using a newly developed version of deGradFP that allows the rapid degradation of GFP-tagged proteins in cultured cells after addition of Shield-1 reagent (Bobkov et al., 2020).
This project is funded by the Deutsche Forschungsgemeinschaft (DFG, grant BR2102/8).