Main Content
Signaling networks in the ovarian cancer microenvironent
Ovarian carcinoma ranks fifth as the cause of death from cancer in women. Most patients with high grade serous ovarian adenocarcinoma (HGSC), the most common tumor subtype, present with advanced stage disease and disseminated tumor masses. Although most cancers are highly sensitive to first-line adjuvant chemotherapy, the disease has an overall 5-year survival rate of less than 40%. These facts clearly attest to the malicious nature of ovarian HGSC and identify this cancer as a major health issue world-wide. Several features characteristic of ovarian HGSC contribute to its fatal nature, including the shedding of tumor cells at a very early stage, their metastatic spread to other pelvic and peritoneal organs via the peritoneal fluid and the tumor-promoting and immune suppressive effect of the peritoneal tumor environment, frequently formed by the malignancy-associated ascites building up in the peritoneal cavity.
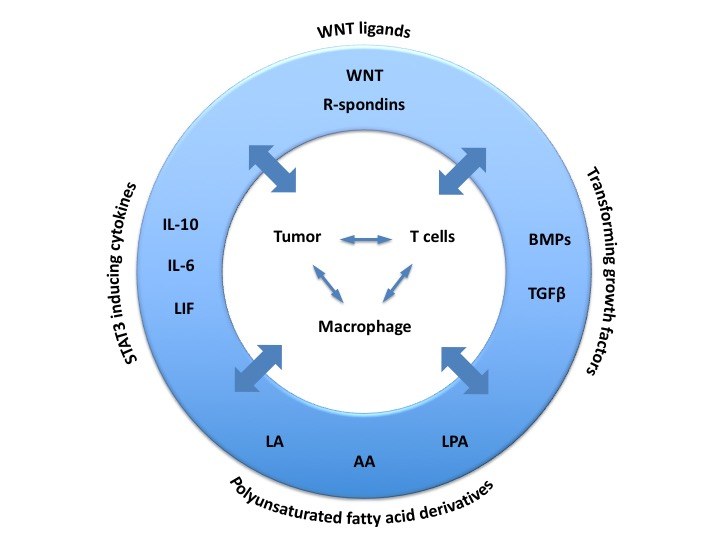
The peritoneal ascites of HGSC patients represents a unique experimental system, since it not only allows for the isolation of large numbers of primary tumor cells and immune cells from ascites, but also provides the opportunity to culture these cells in a medium similar to their in vivo environment, i.e. autologous ascites fluid. In cooperation with the Gynecological Oncology Group (Silke Reinartz) and the Clinic for Gynecology (Uwe Wagner) in Marburg we have therefore focused our attention on both the malignancy-associated ascites and the omentum as the major site of metastatic dissemination. We have integrated our own work and published observations to develop a model that postulates that chemotherapy (CT) failure can arise from the survival of a small number of non-adherent tumor cells shed from solid tumor masses that later on adhere to peritoneal organs, invade, proliferate and form metastatic lesions, and that macrophages (TAMs) play a pivotal role at different stages by promoting adherence, invasion and growth. Soluble protein and lipid mediators play essential roles in the tumor environment. We have investigated their cellular origins, targets and clinical relevance in ovarian HGSC and constructed an extensive proteotranscriptome-derived network of autocrine and paracrine signaling pathways bewteen tumor cells, immune cells and resident host cells of the omentum. We expect that this work will promote our understanding of ovarian cancer biology and the development of novel therapeutic approaces.
This project is carried out within a consortium of several research groups at the Faculty of Medicine in Marburg, who have joined forces to establish OvRA, the Ovarian Cancer Research Alliance at Marburg University.