Hauptinhalt
2009
Inhalt ausklappen Inhalt einklappen APPL. PHYS. A: "Thermally activated dewetting of organic thin films: the case of pentacene on SiO2 and gold"
D. Käfer, Ch. Wöll, G. Witte
Applied Physics A 95 (1), 273-284 (2009), DOI: 10.1007/s00339-008-5011-3
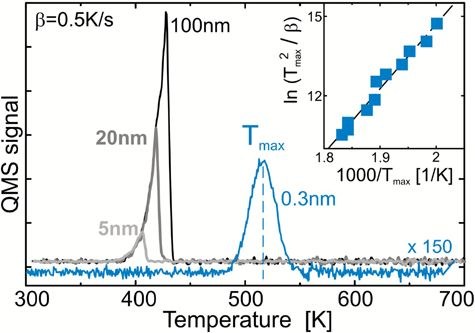
The morphology of pentacene organic thin films deposited on SiO2 and Au(111) surfaces using organic molecular beam deposition (OMBD) has been characterized by a multi-technique approach. Among the techniques applied were X-ray photoelectron spectroscopy (XPS), atomic force microscopy (AFM), scanning electron microscopy (SEM) and thermal desorption spectroscopy (TDS). Our rather detailed studies reveal that on both substrates the growth is strongly influenced by dewetting and islanding phenomena, yielding very rough surfaces. Surprisingly, substantial changes in the morphology were observed also after deposition on room-temperature samples on a time scale of several hours. The rather extensive set of in situ XPS data was analyzed in the framework of a simple model, which allows us to derive rather detailed information on the roughness parameters.Inhalt ausklappen Inhalt einklappen LANGMUIR: "Molecular mechanisms of electron-induced cross-linking in aromatic SAMs"
A. Turchanin, D. Käfer, M. El-Desawy, C. Wöll, G. Witte, A. Gölzhäuser
Langmuir 25 (13), 7342-7352 (2009), DOI: 10.1021/la803538z
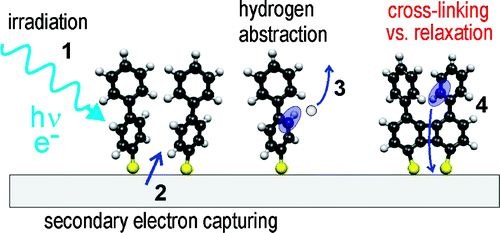
When aromatic self-assembled monolayers (SAMs) are electron-irradiated, intermolecular cross-links are formed and the SAMs transform into carbon nanosheets with molecular thickness. These nanosheets have a very high mechanical stability and can withstand temperatures above 1000 K. In this report, we investigate the electron induced cross-linking of 1,1′-biphenyl-4-thiol (BPT) SAMs on gold by combining X-ray photoelectron spectroscopy (XPS), X-ray absorption spectroscopy (NEXAFS), thermal desorption spectroscopy (TDS), and UV photoelectron spectroscopy (UPS). The experimental data were acquired as a function of electron dose and temperature and compared with quantum chemical calculations. Details of the intermolecular cross-linking, the microstructure of cross-linked films, and their structural transformations upon heating were obtained to derive a view of the mechanisms involved. Our analysis shows that room-temperature electron irradiation causes a lateral cross-linking via the formation of C−C linked phenyl species as well as a new sulfur species. The thermal stability of the BPT films increases with the electron dose and saturates at ∼50 mC/cm2. Nevertheless, nonlinked fragments in the thermal desorption spectra indicate an incomplete cross-linking even at high doses, which can be attributed to steric reasons and quenching due to the reduced band gap of partially linked molecules. At temperatures above 800 K, all sulfur species are thermally desorbed, while the remaining film reveals an onset of carbonization.Inhalt ausklappen Inhalt einklappen INSTR. EXP. TECH.: "A microwave discharge source operating at pressures of several atmospheres"
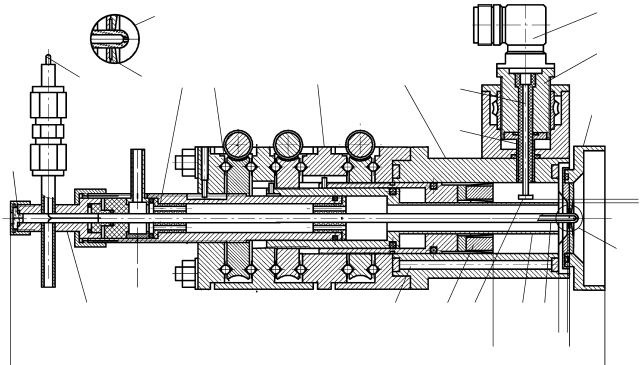
V. M. Akimov, G. Witte, L. I. Kolesnikova, L. Y. Rusin, J. P. Toennies
Instruments and Experimental Techniques 52 (3), 394-399 (2009) • DOI: 10.1134/S0020441209030166
The design of a microwave source in which a discharge is initiated by an electromagnetic surface wave at 2.45 GHz is described. A stable discharge was supported at a gas pressure p0 exceeding the atmospheric pressure in He, N2, and in H2-Ar, H2-He, and O2-He mixtures in a 2-mm inner diameter quartz tube with a 0.15-mm diameter nozzle at a 50- to 115-W microwave power. A degree of dissociation of up to 80% was reached for pure H2 at p0 = 6 Torr and a 6% mixture of H2 and He at p0 = 50 Torr. When p0 increases to 19 Torr for H2 and to 300 Torr for the mixture, the hydrogen-atom beam intensity, in spite of a decrease in the degree of dissociation, increases due to narrowing of the beam particle velocity distribution.