Main Content
Molecular Dynamics Simulations in Individual Mesopores
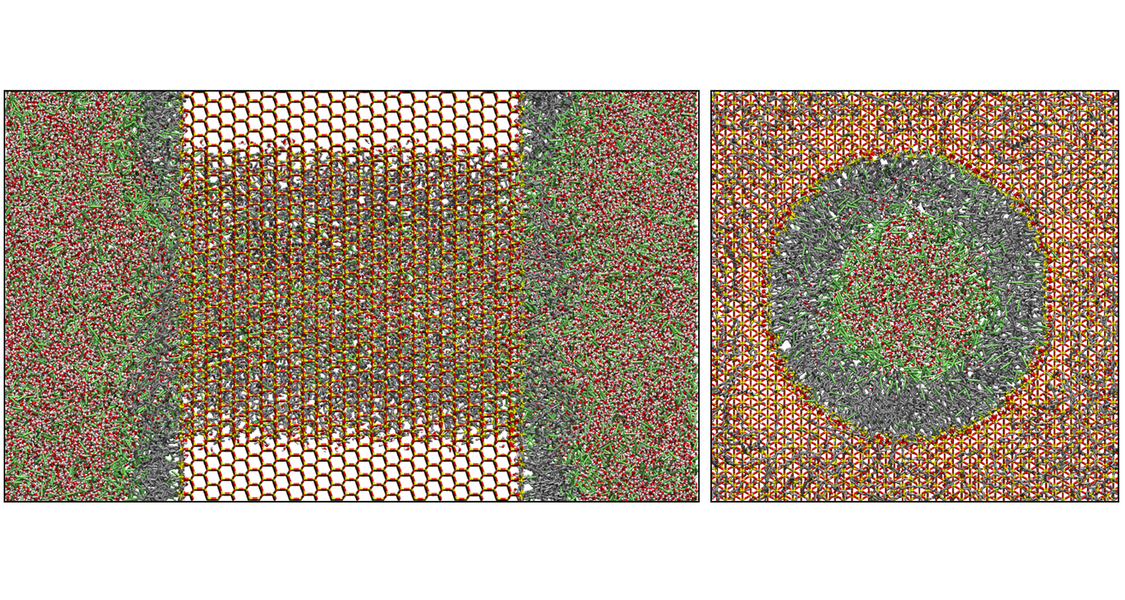
The structure, dynamics, and mobility at the solid−liquid interface play an important role in many processes involving adsorption, catalysis, and separation. We use molecular dynamics (MD) simulations to elucidate the mechanisms of mass transport, retention, and selectivity in the two most relevant liquid chromatography techniques: hydrophilic interaction liquid chromatography (HILIC) and reversed-phase liquid chromatography (RPLC). MD simulations offer the possibility to gain a detailed molecular-level picture of the processes at the solid−liquid interface, which primarily take place inside the mesoporous space of the chromatographic bed (packed porous particles or monoliths) where mass transport is diffusion-limited. Data can be obtained on the spatial distribution, local lateral mobility, hydrogen-bonding structure, orientation, residence times, and local environment of solvent and analyte molecules.
In HILIC, a polar stationary phase (often bare silica) is equilibrated with an aqueous acetonitrile (ACN) mobile phase. Water (W) molecules from the ACN-rich mobile phase accumulate at the bare silica surface and form a W-rich layer. MD simulations (Figure 1) show that the W density decreases from the silica surface to the bulk liquid phase in the pore center. Three regions can be identified in the mesopore: (I) the rigid W layer, which consists mostly of W molecules that are hydrogen-bonded to the silica surface (surface-adsorbed W molecules), (II) the diffuse W layer, whose W molecules are hydrogen-bonded to surface-adsorbed W molecules, and (III) the bulk liquid region, whose composition matches those of the mobile phase. The hydrogen-bonding network in the vicinity of the polar silica surface strongly restricts the diffusive mobility of solvent and analyte molecules in the direction parallel to the surface (surface diffusion) and perpendicular to the surface.
In RPLC, a hydrophobic stationary phase, consisting of alkyl chains (usually C18) that are chemically bonded to the silica surface, is equilibrated with an aqueous ACN or methanol mobile phase. Analogous to HILIC, three regions can be identified in the mesopore (Figure 2): (I) the bonded-phase region, where the overall solvent density is strongly depleted, (II) the interface region, where ACN molecules accumulate at the flexible chain ends, and (iii) the bulk liquid region (III). The ACN-rich layer is referred to as the ACN ditch. In contrast to the W-rich layer in HILIC, the ACN ditch constitutes a high-mobility region so that the surface diffusion of analyte molecules in the ACN ditch (II) is larger than their diffusive mobility in the bulk liquid region (III).
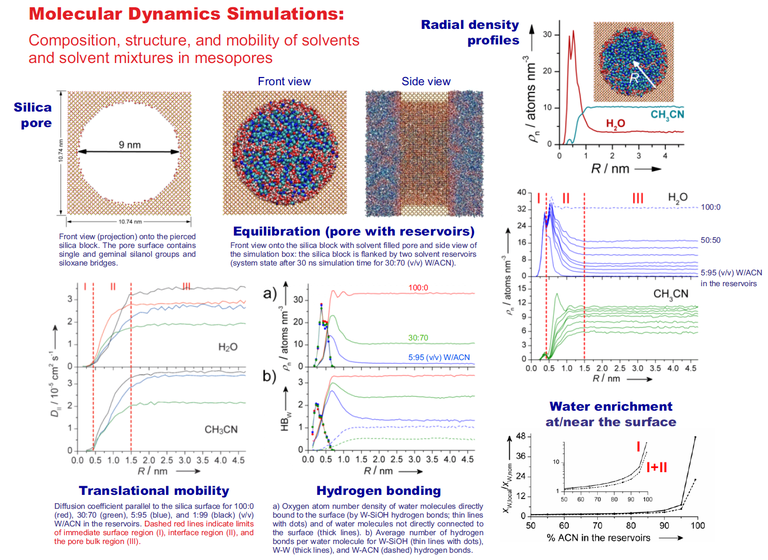

Figure 2. Molecular-level picture of surface diffusion at RPLC-interfaces (planar silica surface modified with C18 chains and a W/ACN mobile phase). In the equilibrated system (left panel), ACN is enriched in the interface region II between the hydrophobic bonded phase and the water-rich mobile phase and forms an ACN-rich high-mobility region, the ACN ditch. ACN and analyte molecules (here benzene) show an increased lateral mobility in the ACN ditch (right panel), which originates from the ACN-rich solvent composition in region II. The snapshots show benzene and its local environment in regions I and II.
Highlighted publications:
- J. Rybka, A. Höltzel, U. Tallarek
Surface diffusion of aromatic hydrocarbon analytes in reversed-phase liquid chromatography.
Journal of Physical Chemistry C 2017, 121, 17907–17920. DOI: 10.1021/acs.jpcc.7b04746
- S.M. Melnikov, A. Höltzel, A. Seidel-Morgenstern, U. Tallarek
A molecular dynamics view on hydrophilic interaction chromatography with polar-bonded phases: Properties of the water-rich layer at a silica surface modified with diol-functionalized alkyl chains.
Journal of Physical Chemistry C 2016, 120, 13126–13138. DOI: 10.1021/acs.jpcc.6b03799
- S.M. Melnikov, A. Höltzel, A. Seidel-Morgenstern, U. Tallarek
Adsorption of water-acetonitrile mixtures to model silica surfaces.
Journal of Physical Chemistry C 2013, 117, 6620–6631. DOI: 10.1021/jp312501b
- S.M. Melnikov, A. Höltzel, A. Seidel-Morgenstern, U. Tallarek
A molecular dynamics study on the partitioning mechanism in hydrophilic interaction chromatography.
Angewandte Chemie International Edition 2012, 51, 6251–6254. DOI: 10.1002/anie.201201096